
AP Chemistry Summer Work
Hello and welcome to AP Chemistry!
In order to best set us up for success this coming year, please complete this summer
work.
On the AP Chemistry Test, you will be provided with a specific Periodic Table and
Reference Sheet. Both of those documents are attached in this packet. We will use these
throughout the year in order to increase our familiarity with them. Take some time this
summer to familiarize yourself with them. The rest of the packet is both notes and
practice problems. You will have to complete the entire packet over the summer.
I highly recommend that you attend the AP summer camp this summer at the
University of Memphis. The AP Chem sessions are offered 6/20-6/23.

Periodic Table Refresher
● “Periods” = Rows, “Groups” = columns.
● Metals make up the majority of the elements on
the periodic table and are located towards the
left/bottom (red & yellow in the image at right).
Nonmetals are in the upper right corner (blue).
The diagonal strip of elements between them are
metalloids (purple).
● The “block” (s, p, d, or f) represents the
orbital shape of the last added electron.
● The “Main groups” are the s & p blocks.
● The “Transition metals” are in d block,
which contain the majority of common “hard”
metals. “Inner Transition” metals are in the f
block (bottom section). Several of these are
radioactive. We don’t generally study these.
● Families are sets of elements, often in a single column/group, that demonstrate similar properties and
reactions. Note: There was an old style of labeling the groups (Main groups were 1A, 2A, then 3A-8A
after the transition metals), but the new style of labeling groups is simply 1-18.
● Below are the most common “Families” of the periodic table:
○ Alkali Metals: Group 1 (1A in the
old style)
○ Alkaline Earth Metals: Group 2
(2A)
○ Transition Metals: Groups 3-12
(3B-2B)
○ Halogens: Group 17 (7A)
○ Noble Gases: Group 18 (8A)
You should be familiar with the charges, reactivity, and
general features of the five main chemical families.

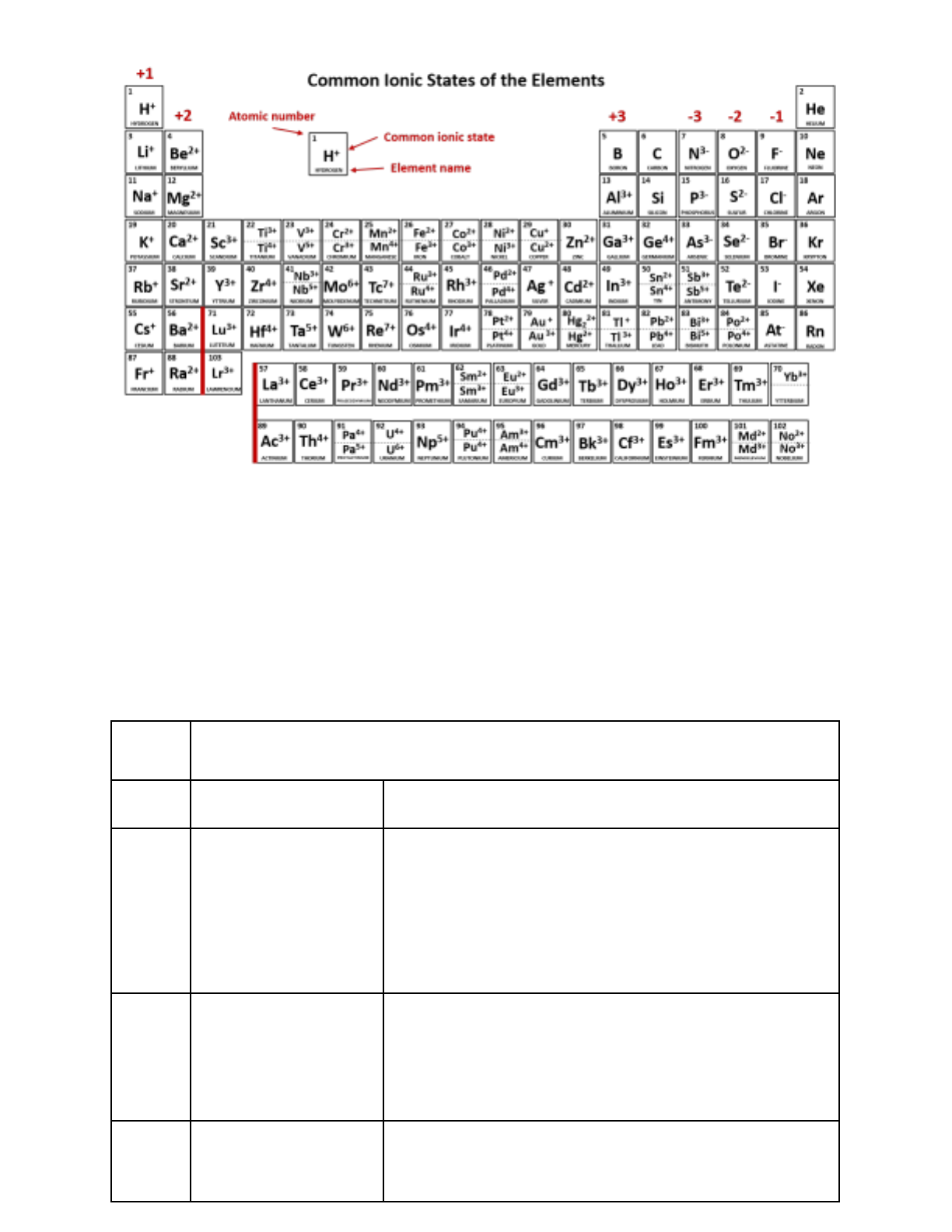
Main Group Ions
The charges that elements form when reacting and forming bonds are key to understanding their behavior.
Elements on the far left of the periodic table tend to form positive ions; elements on the far right tend to form
negative ones. However, there are many exceptions. Keep this information as a reference:
All-Positive Groups
Groups with Both
All-Negative Groups
+1 (-1)
+2
+3 +4 / -4
+5 / -3
-2
-1
Group 1 (1A)
Group 2 (2A)
Group 13
Group 14
(3A)
(4A)
Group 15
(5A)
Group 16
(6A)
Group 17
(7A)
Alkali
Metals (and
Hydrogen)
Alkaline
Earth Metals
Boron
Carbon
Group
Group
Nitrogen
Group
Oxygen
Group
Halogens
Transition Metal Ions
Transition metals (all in the “d” block) and post-transition metals (those below the metalloids in the “p” block)
form exclusively positive ions but may form different charges depending on the circumstance. Roman numerals
after the name indicate the charge.
● Fe, Iron (II) or (III): +2 or +3 ● Cu, Copper (I)
or (II): +1 or +2 ● Hg, Mercury (I) or (II): +1 or
+2 ● Sn, Tin (II) or (IV): +2 or +4 ● Pb, Lead (II)
or (IV): +2 or +4 ● Co, Cobalt (II) or (IV): +2 or
+4 ● Mn, Manganese (II) or (IV): +2 or +4
● Cr, Chromium (II) or (III): +2 or +3 ●
NO ROMAN NUMERALS:
○ Ag, Silver +1
○ Zn, Zinc +2
○ Cd, Cadmium +2
Polyatomic Ions
Polyatomic ions are made of several atoms covalently bonded together that then act as a single,
unbreakable unit. Their names, formulas and charges are memorized. Familiarize yourself
with these polyatomic ions. Make flashcards with the ‘name’ on one side & the ‘ion’ on the
other side (Don’t forget the charge!) These are not the only polyatomic ions you will encounter,
they are just the most common.
Charg
e
Polyatomic Ions
1+
Ammonium NH
4
+
Hydronium H
3
O
+
1-
Perchlorate ClO
4
-
Chlorate ClO
3
-
Chlorite ClO
2
-
Hypochlorite ClO
-
-
Cyanide CN
-
Acetate CH
3
COO
-
Nitrate NO
3
-
Bicarbonate HCO
3
-
Nitrite NO
2
-
Hydroxide OH
-
2-
Carbonate CO
3
2-
Chromate CrO
4
2-
Sulfate SO
4
-2
Oxalate C
2
O
4
-2
Sulfite SO
3
-2
Dichromate Cr
2
O
7
-2
3-
Phosphate PO
4
-3

Diatomic Molecules
All elements can be found in compounds, in which case their subscripts and ratios will be
determined by the bonding patterns of the particular compound. However, when in their pure
elemental state, some elements are monatomic -- meaning they are found as single atoms (such
as He and Ar) while others are not. Diatomic elements are usually found in bonded pairs of two:
BrINClHOF: Br
2
I
2
N
2
Cl
2
H
2
O
2
F
2
Part 4: Significant Figures in Measurement and Calculations
A successful chemistry student habitually labels all numbers, because the unit is important. Also
of great importance is the number itself. Any number used in a calculation should contain only
figures that are considered reliable, which are called “significant figures”. Chemical calculations
involve numbers representing actual measurements. In a measurement, significant figures in a
number consist of: Figures (digits) definitely known + One estimated figure.
There are rules for 1) performing measurements & gathering data in significant
figures and 2) doing calculations in significant figures.
Measuring with Sig Figs
Find the nearest marked number for the
item
you’re measuring. That is your only
“definitely
known” number. Then estimate on more
digit of
precision. A correct reading of this
measurement would be “29.1” or “29.2”.
Just “29” would not be correct because it’s missing the estimated digit. “29.15” would not be
correct because that’s estimating too many digits; the ruler does not give that much precision.
However, if we get a better rule
with
more precise markings, we
can read
to an extra “sig fig”. Here, the

markings show us the object reaches, for
sure, the 29.2 mark. Then we estimate
how far between the .2 and .3 mark it
falls. A correct reading of this
measurement would be “29.24” or “29.25”. If the above landed directly on the line, we
would still report an extra digit; we would simply report it as a “0”, If you forget to do that,
you’re reporting that you used a less precise tool than you actually used.
Calculating with Sig Figs
● When doing addition or subtraction, the answer should have the same precision as
the least precise measurement (value) used in the calculation.
○ Since the level of precision is related to the number of decimal places in the
measured value, you then round to the least number of decimal places.
○ 1.586 + 2.31 = 3.896 = 3.90 since 2.31 has only two decimal places.
● When doing multiplication or division, the answer should have the same number of
significant figures as the measured value with the least number of significant figures. ○
You must count the number of sig figs in each original value and then round the answer
to the lowest number.
○ 16.156 / 2.72 = 5.93970588 = rounds to 5.94 since 2.72 has three sig figs.
● How to round:
○ If the figure to be dropped is less than 5, simply eliminate it. (2.42 => 2.4) ○ If the
figure to be dropped is 5 or greater, eliminate it and raise the preceding figure by 1.
(2.47 => 2.5)
○ In the case that the preceding figure is a 9 and is rounded up to a 10, be sure to
keep that last 0.
■ Ex: 0.598 needs to be rounded to 2 sig figs. 8 is dropped and rounds the
0.59 to a 0.60. Report it as 0.60, NOT as 0.6.
Counting Sig Figs
● All non-zero numbers are significant (4.53 has three sig figs).
● Zeros in the middle of a number are significant (4.503 has four sig figs). ● Trailing
zeros (Zeros at the end of a number) are significant (4.50 has three sig figs) because the 0’s
are holding the place of a level of precision.
● Leading zeros are NOT significant. In 0.070, there are only two sig figs; the others are
placeholders.

Practice Review Questions
Answer the following questions.
1. What family is F in?
2. What does it mean if an atom is diatomic?
3. How many significant figures are in each of the following:
a. 11
b. 10
c. 0.001
d. 170018.9
4. What are the rules for significant figures when multiplying or dividing?
5. What are the rules for significant figures when adding or subtracting?
6. Calculate the molarity of a solution (M=mol/L) with appropriate significant figures if 0.17
moles of NaCl were dissolved in 1.23 L of solution.
7. What charge do all alkaline earth metals have?
8. Some transition metals always have the same charge. Since these always have the same
charge, they will not have roman numerals included in their nomenclature. List the charge
of these transition elements below.
a. Silver
b. Zinc
9. What are the diatomic atoms?

STOICHIOMETRY
The following flow chart may help you work on stoichiometry problems. Remember to pay careful
attention to what you are given, and what you are trying to find. For each of the problems, use appropriate
significant figures.
1. Fermentation is a complex chemical process of making wine by converting glucose into ethanol and
carbon dioxide:
C
6
H
12
O
6
(s) → 2 C
2
H
5
OH (l) + 2 CO
2
(g)
A. Calculate the mass of ethanol produced if 500.0 grams of C
6
H
12
O
6
reacts completely.

2. Consider the reaction of zinc metal with hydrochloric acid, HCl(aq).
A. Write the equation for this reaction, then balance the equation.
B. Calculate the moles of HCl needed to react completely with 8.25 moles of zinc.
C. Calculate the volume of hydrogen gas produced at STP if 25.0 grams of HCl react completely.
3. If you dissolve lead(II) nitrate and potassium iodide in water they will react to form lead(II) iodide and
potassium nitrate.
A. Write the equation for this reaction, then balance the equation.
B. What type of reaction is this?
C. Calculate the grams of lead(II) iodide that can be produced from 75.00 grams of potassium iodide.

Lewis Dot Structures Notes
Step 1: Determine the total number of valence electrons.
Step 2: Write the skeleton structure of the molecule.
Step 3: Use two valence electrons to form each bond in the skeleton structure.
Step 4: Try to satisfy the octets of the atoms by distributing the remaining valence electrons as nonbonding
electrons.
Step 5: If there aren’t enough electrons to satisfy the octet rule, form double or triple bonds.
The first step in this process involves calculating the number of valence electrons in the molecule or ion. For a
neutral molecule this is nothing more than the sum of the valence electrons on each atom. If the molecule carries
an electric charge, we add one electron for each negative charge or subtract an electron for each positive charge.
Example: Let's determine the number of valence electrons in the chlorate (ClO
3
-
) ion.
A chlorine atom (Group VIIA) has seven valence electrons and each oxygen atom (Group VIA) has six valence
electrons. Because the chlorate ion has a charge of -1, this ion contains one more electron than a neutral ClO
3
molecule. Thus, the ClO
3
-
ion has a total of 26 valence electrons.
ClO
3
-
: 7 + 3(6) + 1 = 26
The second step in this process involves deciding which atoms in the molecule are connected by covalent bonds.
The formula of the compound often provides a hint as to the skeleton structure. The formula for the chlorate ion,
for example, suggests the following skeleton structure.
The third step assumes that the skeleton structure of the molecule is held together by covalent bonds. The valence
electrons are therefore divided into two categories: bonding electrons and nonbonding electrons. Because it takes
two electrons to form a covalent bond, we can calculate the number of nonbonding electrons in the molecule by
subtracting two electrons from the total number of valence electrons for each bond in the skeleton structure.
26 total valence electrons - 6 bonding electrons= 20 remaining electrons
There are three covalent bonds in the most reasonable skeleton structure for the chlorate ion. As a result, six of the
26 valence electrons must be used as bonding electrons. This leaves 20 nonbonding electrons in the valence
shell.The remaining valence electrons are now used to satisfy the octets of the atoms in the molecule. Each oxygen
atom in the ClO
3
-
ion already has two electrons the electrons in the Cl-O covalent bond. Because each oxygen
atom needs six nonbonding electrons to satisfy its octet, it takes 18 nonbonding electrons to satisfy the three
oxygen atoms. This leaves one pair of nonbonding electrons, which can be used to fill the octet of the central atom.

Lewis Dot Structures Practice Problems
Formula
Total number of valence
electrons
Lewis Dot Structure
CH
4
CO
2
NH
3
H
2
O
Cl
2
BF
3
Remember B does
not follow the
octet rule and
only needs 6
valence electrons

Periodic Trends
Use the infographic from the ACS to answer the following questions.
1. What element in the same period as astatine has the greatest first ionization energy?
2. What element in the same family as magnesium has the lowest first ionization energy?
3. What element in the halogens has the largest atomic radius?
4. What element in the same period as potassium has a smaller atomic mass?
5. What element in the same group as barium has a lesser first ionization energy?
6. What element in the same period as cesium has a smaller atomic mass?
7. What element in the same group as oxygen has the greatest electronegativity?
8. What element in the same period as chlorine has the least electronegativity?
9. What element in the same period as sodium has the greatest first ionization energy?
10. What element in the same family as nitrogen has a smallest atomic radius?

MASTERING
Periodic Trends
Perfect your performance with periodicity!
Important Trend Terms
Eective nuclear charge: the net positive charge from the nucleus that an
electron can “feel” attractions from. The core electrons are said to shield the
valence electrons from the full attractive forces of the protons in the nucleus.
Shielding: core (nonvalence) electrons shield the valence electrons
from the full attractive forces of the protons in the nucleus.
Electron-electron repulsions: due to their like charges, electron
pairs orient themselves as far away as possible from each other,
causing the electron cloud to expand (justifies trends across a period).
1. Atomic Radius
Atomic radius increases
Atomic radius increases
Atomic radius is the distance from the atom’s nucleus to the outer edge of the
electron cloud.
In general, atomic radius decreases across a period and increases down a group.
Across a period, eective nuclear charge increases as electron shielding remains constant.
A higher eective nuclear charge causes greater attractions to the electrons, pulling the
electron cloud closer to the nucleus which results in a smaller atomic radius.
Down a group, the number of energy levels (n) increases, so there is a greater distance between
the nucleus and the outermost orbital. This results in a larger atomic radius.
2. Ionic Radius
Metals Nonmetals
Ionic radius increases
Ionic radius increases
Ionic radius is the distance from the nucleus to the outer edge of the electron cloud
of an ion.
The same trend of atomic radius applies once you divide the table into metal and
nonmetal sections.
A cation has a smaller radius than its neutral atom because it loses valence electrons. The “new”
valence shell is held closer to the nucleus, resulting in a smaller radius for the cation.
An anion has a larger radius than the neutral atom because it gains valence electrons. There are
added electron/electron repulsions in the valence shell that expand the size of the electron cloud,
which results in a larger radius for the anion.
3. Ionization Energy
IE increases
IE increases
Ionization energy (IE) is the energy required to remove the highest-energy electron
from a neutral atom.
In general, ionization energy increases across a period and decreases down a group.
Across a period, eective nuclear charge increases as electron shielding remains constant.
This pulls the electron cloud closer to the nucleus, strengthening the nuclear attraction to the
outer-most electron, and is more dicult to remove (requires more energy).
Down a group, the number of energy levels (n) increase and the distance is greater between
the nucleus and highest-energy electron. The increased distance weakens the nuclear attraction
to the outer-most electron, and is easier to remove (requires less energy).
4. Electronegativity
Electronegativity increases
Electronegativity
increases
F
Electronegativity is the measure of the ability of an atom in a bond to attract
electrons to itself.
Electronegativity increases across a period and decreases down a group.
Towards the left of the table, valence shells are less than half full, so these atoms (metals) tend
to lose electrons and have low electronegativity. Towards the right of the table,
valence shells are more than half full, so these atoms (nonmetals) tend to gain electrons and
have high electronegativity.
Down a group, the number of energy levels (n) increases, and so does the distance between
the nucleus and the outermost orbital. The increased distance and the increased
shielding weaken the nuclear attraction, and so an atom can’t attract electrons as strongly.
Fluorine is the most electronegative element, whereas francium is the least
electronegative element.

1 18
PERIODIC TABLE OF THE ELEMENTS
1 2
H
He
1.008
2 13 14 15 16 17
4.00
3
Li
6.94
11
Na
22.99
4
Be
9.01
12
Mg
24.30
3 4 5 6 7 8 9 10 11 12
5
B
10.81
13
Al
26.98
6
C
12.01
14
Si
28.09
7
N
14.01
15
P
30.97
8
O
16.00
16
S
32.06
9
F
19.00
17
Cl
35.45
10
Ne
20.18
18
Ar
39.95
19
K
39.10
20
Ca
40.08
21
Sc
44.96
22
Ti
47.87
23
V
50.94
24
Cr
52.00
25
Mn
54.94
26
Fe
55.85
27
Co
58.93
28
Ni
58.69
29
Cu
63.55
30
Zn
65.38
31
Ga
69.72
32
Ge
72.63
33
As
74.92
34
Se
78.97
35
Br
79.90
36
Kr
83.80
37
Rb
85.47
38
Sr
87.62
39
Y
88.91
40
Zr
91.22
41
Nb
92.91
42
Mo
95.95
43
Tc
44
Ru
101.07
45
Rh
102.91
46
Pd
106.42
47
Ag
107.87
48
Cd
112.41
49
In
114.82
50
Sn
118.71
51
Sb
121.76
52
Te
127.60
53
I
126.90
54
Xe
131.29
55
Cs
132.91
56
Ba
137.33
57-71
72
Hf
178.49
73
Ta
180.95
74
W
183.84
75
Re
186.21
76
Os
190.23
77
Ir
192.22
78
Pt
195.08
79
Au
196.97
80
Hg
200.59
81
Tl
204.38
82
Pb
207.2
83
Bi
208.98
84
Po
85
At
86
Rn
87
Fr
88
Ra
89-103
104
Rf
105
Db
106
Sg
107
Bh
108
Hs
109
Mt
110
Ds
111
Rg
112
Cn
113
Nh
114
Fl
115
Mc
116
Lv
117
Ts
118
Og
57 58 59 60 61 62 63 64 65 66 67 68 69 70 71
*Lanthanoids
La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu
138.91
89
†Actinoids
Ac
140.12 140.91 144.24 150.36 151.97 157.25 158.93 162.50 164.93 167.26 168.93 173.05 174.97
90
Th
232.04
91
Pa
231.04
92
U
238.03
93
Np
94
Pu
95
Am
96
Cm
97
Bk
98
Cf
99
Es
100
Fm
101
Md
102
No
103
Lr
AP Chemistry Periodic Table of the Elements
1 of 1
© 2020 College Board
*
†

00762-115-CED-Chemistry_Appendix.indd 232
13/04/19 5:20 PM
© 2020 College Board
1 of 2
AP Chemistry Equations and Constants

AP Chemistry Equations and Constants
00762-115-CED-Chemistry_Appendix.indd 233
M
å
å
å
D
å
D
åå
R
E
cell
=
o
T
EQ
cell
ln
nF
D
D
13/04/19 5:20 PM
2 of 2
© 2020 College Board
