
Pharma 2020: Challenging business models
Which path will you take?
Pharmaceuticals and Life Sciences

Table of contents
Pharma 2020: The vision #
Pharma 2020: The vision
Which path will you take?*
Pharmaceuticals
*connectedthinking
Pharma 2020: Virtual R&D 1
Pharma 2020: Virtual R&D
Which path will you take?
Pharmaceuticals and Life Sciences
Pharma 2020: Marketing the future
Which path will you take?
Pharmaceuticals and Life Sciences
Previous publications in this series include:
This report, published in June 2008,
explores opportunities to improve the R&D
process. It proposes that new technologies
will enable the adoption of virtual R&D; and
by operating in a more connected world the
industry, in collaboration with researchers,
governments, healthcare payers and
providers, can address the changing needs
of society more effectively.
Published in February 2009, this paper
discusses the key forces reshaping the
pharmaceutical marketplace, including
the growing power of healthcare payers,
providers and patients, and the changes
required to create a marketing and sales
model that is fit for the 21st century. These
changes will enable the industry to market
and sell its products more cost-effectively,
to create new opportunities and to generate
greater customer loyalty across the
healthcare spectrum.
Published in June 2007, this paper
highlights a number of issues that will
have a major bearing on the industry by
2020. The publication outlines the changes
we believe will best help pharmaceutical
companies realise the potential the future
holds to enhance the value they provide to
shareholders and society alike.
“Pharma 2020: Challenging business models” is the fourth paper in the Pharma 2020 series on the future of the pharmaceutical industry to be
published by PricewaterhouseCoopers. This publication highlights how Pharma’s fully integrated business models may not be the best option for the
pharma industry in 2020; more creative collaboration models may be more attractive. This paper also evaluates the advantages and disadvantages of
the alternative business models and how each stands up against the challenges facing the industry.
All these publications are available to download at: www.pwc.com/pharma2020
Pharma 2020:
Challenging business models
Table of contents
Introduction 1
Profiting alone versus profiting together 1
Harking back to the future 2
Reading the signs 2
Broadening the value proposition and managing the value chain 4
Choosing between different collaborative models 6
The federated model•
The virtual variant of the federated model•
The venture variant of the federated model•
The fully diversified model•
Charting a successful course 12
Conclusion 13
Acknowledgements 15
References 17

Table of contents

Pharma 2020:
Challenging business models
1
Introduction
The pharmaceutical marketplace
is undergoing huge changes, as
we indicated in “Pharma 2020:
The vision”, the White Paper
PricewaterhouseCoopers* published in
June 2007.
1
These changes will have a
major bearing on the kind of business
models pharmaceutical companies
need to employ.
Most Big Pharma companies have
traditionally done everything from research
and development (R&D) through to
commercialisation themselves. But we
predict that, by 2020, this model will
no longer work for many organisations.
If they are to prosper, they will need to
improve their R&D productivity, reduce
their costs, tap the potential of the
emerging economies and switch from
selling medicines to managing outcomes
– activities few, if any, companies can
accomplish on their own.
Even the largest pharmaceutical
companies will have to collaborate with
other organisations to develop effective
new medicines more economically,
help patients manage their health and
ensure that the products and services
they provide really make a difference.
Moreover, they may have to step far
outside the sector to find some of the
partners they need.
We believe that two principal business
models – federated and fully diversified
– will emerge, as Pharma prepares for
the future. We also think that the current
economic downturn will accelerate
the shift to these new models, both by
reinforcing one of the key causal factors
– the pressure on healthcare payers
to maximise the value they get for the
money they spend – and by opening up
new opportunities to build or buy the
networks that will be required.
In the following pages, we shall look
at the main trends dictating the need
for a more collaborative approach. We
shall also evaluate the advantages and
disadvantages of the alternative business
models and how each stands up against
the challenges facing the industry.
Profiting alone versus
profiting together
Big Pharma’s traditional business model
hinges on the ability to identify promising
new molecules, test them in large clinical
trials and promote them with an extensive
marketing and sales presence (see
sidebar, What is a business model?). In
the predominant version of this model, a
single company may employ contractors
to supplement its own efforts, but it
seeks to generate profits on its own. In
essence, it pursues what might be called
a “profit alone” path.
But, by 2020, the strategy of
singlehandedly placing big bets on a
few molecules, marketing them heavily
and turning them into blockbusters will
not suffice. As J.P. Garnier, former chief
executive of GlaxoSmithKline, recently
pointed out, it is a “business model
where you are guaranteed to lose your
entire book of business every 10 to
12 years”.
2
More importantly still, it is a business
model that will no longer meet the
market’s needs. Management guru
Clay Christensen has convincingly
demonstrated how disruptive
innovations in various industries have
dismantled the prevailing business
model, by enabling new players to
target the least profitable customer
segments and gradually move upstream
until they can satisfy the demands of
every customer – at which point the old
business model collapses.
3
Pharma is currently undergoing just
such a period of disruptive innovation.
By 2020, most medicines will be
paid for on the basis of the results
they deliver – and since many factors
influence outcomes, this means that
it will have to move into the health
management space, both to preserve
the value of its products and to avoid
being sidelined by new players. If it is to
make groundbreaking new medicines
for which governments and health
insurers are prepared to pay premium
prices, it will also have to build the
relationships and infrastructure required
to ensure that it can get access to the
outcomes data they collect.
In short, the rules of the game are
shifting dramatically. And, as Michael
G. Jacobides, Associate Professor of
Strategic and International Management
at the London Business School, notes,
when an entire “industry architecture”
is transformed, it is not only “who does
what” that changes, it is also “who
takes what”.
4
By 2020, no pharmaceutical
company will be able to “profit
alone”. It will, rather, have to “profit
together”, by joining forces with a
wide range of organisations, from
What is a business model?
The term “business model” is used
to encompass a wide range of formal
and informal descriptions of the core
elements of a business. We have
used the term in the following sense:
“A company’s business model is the
means by which it makes a profit –
how it addresses its marketplace, the
offerings it develops and the business
relationships it deploys to do so.”
*‘PricewaterhouseCoopers’ refers to the network of member firms of PricewaterhouseCoopers International Limited, each of which is a separate and
independent legal entity.

2 PricewaterhouseCoopers
academic institutions, hospitals and
technology providers to companies
offering compliance programmes,
nutritional advice, stress management,
physiotherapy, exercise facilities, health
screening and other such services.
Harking back to the
future
Of course, some pharmaceutical
companies have already tried to
collaborate with other organisations.
Rhone-Poulenc Rorer (now part of
sanofi-aventis) created RPR Gencell, the
world’s first biotechnology network, in
1994.
5
Many of the largest companies
also established disease management
programmes in the 1990s, although
most of them were not very successful
– primarily because healthcare
payers were sceptical about industry-
sponsored disease management.
6
So we are not suggesting that the
differences between these early efforts
and the business models that are likely
to prevail in 2020 will be completely
black and white. Nevertheless, we think
that two key differences will apply.
First, the technological and cultural
pre-conditions to facilitate collaboration
are now in place. In the mid-1990s, the
Internet was still in its infancy and many
of the tools that enable collaboration did
not exist. Today, however, such tools are
plentiful and the wider business culture
has changed dramatically. IBM, Apple,
Amazon and their ilk have demonstrated
the power of open platforms,
transformed corporate attitudes
towards networking and shown that it is
possible to reap much richer rewards by
profiting together than by profiting alone
(see sidebar, Apple’s core strategy of
collaboration).
7
Second, by 2020, collaboration
will be a “do or die” requirement
for pharmaceutical companies and
healthcare payers alike. It will be
essential for pharmaceutical companies
to develop effective new medicines
and address the demands of payers
increasingly well equipped to measure
what they are getting for their money;
and essential for payers to cope with
rapidly escalating healthcare costs.
Reading the signs
Various forces are changing the
environment in which Pharma operates
and the relative positions of the different
players in the healthcare arena. These
trends all point towards the need for
much greater collaboration (see Figure 1).
The global healthcare bill is soaring,
as the population ages, new medical
needs emerge and the disease burden
of the developing world increasingly
resembles that of the developed world.
Hence the fact that governments
and health insurers everywhere are
struggling to contain their expenditure.
The issue is further exacerbated by the
current economic turmoil that will put
even greater financial pressure on the
payer community.
Healthcare payers in the industrialised
economies are already mandating
what doctors can prescribe. The
British National Health Service has
also introduced a flexible pricing
scheme under which the prices of
new medicines can be lowered or
lifted, depending on the outcomes
they deliver.
8
And US President
Barack Obama’s administration is
moving towards opening up the US
market to much greater competition
from generics, as well as allowing the
importation of cheaper medications
from “safe” countries.
9
Apple’s core strategy of
collaboration
London Business School Professor
Michael G. Jacobides has recently
argued that successful companies do
not compete in a sector; they shape
the nature of a sector. They redefine
the part of the value chain they
occupy, and keep most of the value-
add through the intelligent design of
their collaboration with others in the
sector.
Thus collaboration is not just a tool
for doing the same things more
effectively. At its most powerful, it
can reshape an entire market, as
Apple has shown. Apple redefined the
mobile music sector by outsourcing
the production of the devices and
accessories, while retaining control of
the iTunes software. In other words,
it recognised that it could make
money by creating and orchestrating
a network of relationships – by
controlling, rather than owning.
Apple used three specific tactics
to change the rules of the game. It
enhanced the mobility of the parts
of the sector in which it has no
presence, by establishing a small
set of suppliers who know that they
can be replaced at any time. It made
itself into a bottleneck, by holding
onto the music format and ensuring
that files compatible with iPod can
only be played on iPod devices.
And it redefined who did what, by
encouraging other companies to
develop accessories rather than
entering the accessories market
itself. This has enabled it to benefit
from the efforts of those that support
its architecture, without making any
capital commitment itself.

Pharma 2020:
Challenging business models
3
The developing world will soon come
under equal pressure. The emerging
economies will experience the most
rapid growth in demand for medicines
over the next 11 years, but many (if not
all) of them will struggle to fund this
demand. The Chinese government has,
for example, undertaken to introduce
a universal healthcare system with a
level of cover that does not exceed
the country’s current economic
development. However, it is hard to see
how the plan will not entail a substantial
increase in China’s healthcare costs.
10
Healthcare payers in both the
developed and developing worlds are
also beginning to measure outcomes
much more carefully and to emphasise
the importance of prevention. By 2020,
they will expect the industry to go
“beyond the medicine” by providing
prophylactics and healthcare packages
designed to help patients manage their
health. Moreover, patients will play a
much bigger role in determining how
they are treated, as the money they
spend on medicines likewise rises
and the Internet gives them access to
more information. Armed with insights
Figure 1: The key trends now emerging and their implications for Pharma
Health and healthcare trends Scientific and technological trends
Pharma will need to go “beyond the
medicine”
R&D will need to go beyond the lab
Trends
Implications
Market trends
The Pharma and healthcare value chains
will become much more intertwined
Business models based on collaboration
• Pharma will be paid for outcomes,
not products
• Outcomes data will drive healthcare
policy
• Prevention will gain a higher healthcare
profile
• Pharma will need to offer “medicine-
plus” packages of care
• Pharma will have to adopt more flexible
pricing strategies
• Pharma will need access to outcomes
data
• Pharma will have to work with
technology vendors to virtualise R&D
• Pharma will need a wider, more
multi-disciplinary skills base
• Pharma will need to expand its
presence in Asia
• Pharma will need to demonstrate “real”
value-for-money
• Pharma will have to work more closely
with the regulators
• Pharma will have to collaborate with
payers and providers to perform
continuous trials
• Pharma will have to collaborate with
numerous service providers to deliver
packages of care
• R&D is becoming more virtualised
• The research base is shifting to Asia
• Remote monitoring is improving rapidly
• The burden of – and bill for – chronic
disease is soaring
• Healthcare payers are establishing
treatment protocols
• Pay-for-performance is on the rise
• The boundaries between different forms
of care are blurring
• Financial constraints on payers are
increasing
• Patients are becoming better informed
• Patients are picking up a bigger share
of the bill
• Demand for personalised medicine is
increasing
• Patients want cures, not treatments
• The emerging markets are becoming
more important
Source: PricewaterhouseCoopers

4 PricewaterhouseCoopers
gleaned from educational websites,
discussion groups and blogs, they will
not only want better, safer medicines,
they will also want a range of satellite
services they can tailor to their
individual needs.
If Pharma is to accommodate these
changes in the marketplace, it will have
to collaborate much more extensively
– as it will, indeed, to capitalise on
some of the scientific and technological
trends that are now emerging. The
research base is shifting, for example.
Non-OECD economies accounted for
18.4% of the world’s R&D in 2005, up
from 11.7% in 1996. The number of
patents filed by Asian researchers also
increased significantly over the same
period, albeit from low levels.
11
So the
industry will have to forge much closer
links with the most reputable centres of
scientific excellence in these countries.
Meanwhile, new technologies are
providing new sources of knowledge.
Home surveillance systems, portable
devices and implants, linked to online
and wireless networks, will facilitate
the monitoring of patients on a real-
time basis outside a clinical setting.
But if Pharma is to get access to the
outcomes data remote monitoring
generates, it will have to collaborate
with the hospitals and clinics that
capture this information.
Technological advances will likewise
enable the virtualisation of large parts
of the R&D process, as we explained in
“Pharma 2020: Virtual R&D”.
12
Some of
the leading pharmaceutical companies
are already exploring the potential of
semantic technologies and computer-
aided molecule design. Various
academic institutes and bioinformatics
firms are also building computer models
of different organs and cells, with the
ultimate aim of creating a “virtual man”.
But developing such a model will require
a monumental collaborative effort far
exceeding that required to complete the
Human Genome Project.
13
The economic case for change is
clear. The decline of revenue growth
and margins result in reduced
shareholder returns which will force
pharmaceutical companies to adapt.
There is a compelling case for increased
collaboration. Delivering drug therapies
to payers and patients in a 2020 world
will require new skills, technologies and
channels - the infrastructure required will
be uneconomic for anyone, other than
the largest players, to build internally.
To sum up, the key social, economic
and technological changes currently
taking place in the pharmaceutical and
healthcare arena will all necessitate the
development of multinational, multi-
disciplinary networks drawing on a
much wider range of skills than Pharma
alone can provide. The constraints
that previously hindered organisations
from collaborating over distance are
simultaneously evaporating – paving the
way for the use of new business models
(see sidebar, Emerging collaborative
networks).
14
In the next sections, we shall
look at the implications of broadening the
value proposition, the various models that
exist and the different opportunities and
risks they present.
Broadening the value
proposition and
managing the value chain
Pharma currently creates value by
developing new medicines (and a
relatively limited number of diagnostics).
Collaborating much more closely with
the key stakeholders in the healthcare
sector will enable the industry both
to expand its remit and to align its
Emerging collaborative networks
Several pharmaceutical firms
have already begun to use more
collaborative models. One such
instance is Lilly, which is currently
transforming itself from a traditional
fully integrated pharmaceutical
company into a fully integrated
pharmaceutical network, so that it can
draw on a wide range of resources
beyond its own walls. Lilly hopes that
teaming up with other organisations
to create virtual R&D programmes
will enable it to get better access to
innovation, reduce its costs, manage
risks more effectively and enhance
its productivity. For example, the
Chorus Project is a virtual organisation
to take molecules quickly to Proof
of Concept. Lilly also uses external
networks comprising third parties such
as Piramal Life Sciences, Hutchison
MediPharma, Suven Life Sciences for
the development of molecules.
Swiss biopharmaceutical development
specialist Debiopharm has pioneered a
more radical approach. The company
in-licenses promising new candidates
from academic institutes and biotech
companies, develops them and then
out-licences them to Big Pharma.
Debiopharm’s successes include three
products with combined global sales
of more than US$2.6 billion in 2007.
Most of the collaborative models that
currently exist are limited to R&D. But
it is easy to envisage various other
permutations, including networks
focusing on different therapeutic
areas and covering everything from
R&D through to sales and marketing;
networks focusing on different
enabling technologies, such as
genomics, proteomics and stem cell
research; and networks focusing
on the management of outcomes in
specific patient segments.

Pharma 2020:
Challenging business models
5
value chain more closely with those of
healthcare payers and providers.
As we indicated in more detail in
“Pharma 2020: Marketing the future”,
the value chains of the three parties
are heavily interdependent. The value
payers generate depends on the
policies and practices of the providers
they use. The value providers generate
depends on the revenues payers raise
and the medicines Pharma makes. And
the value Pharma generates depends
on getting access to the patients whom
providers serve and income from the
payers who fund those providers. Yet
the relationship between the different
players is often quite antagonistic and,
while they continue to clash, they are
struggling to retain their respective
goals.
15
If Pharma broadens its value
proposition, it can begin to close the
gap. Creating feedback loops to capture
outcomes data will help it to establish
a more dynamic relationship with
healthcare payers and providers. So,
too, will building the networks required
to deliver healthcare packages that
encompass a wide range of products
and services from numerous different
suppliers. This will ultimately result in
the convergence of the separate, linear
value chains that exist today and the
emergence of a single, circular value
chain (see Figure 2).
Patient
E
p
i
d
e
m
i
o
l
o
g
i
c
a
l
s
t
u
d
i
e
s
,
A
d
m
i
n
i
s
t
r
a
t
i
v
e
s
e
r
v
i
c
e
s
e
t
c
.
P
r
a
c
t
i
c
e
g
u
i
d
e
l
i
n
e
s
,
c
l
i
n
i
c
a
l
g
u
i
d
a
n
c
e
,
p
h
a
r
m
a
c
o
e
c
o
n
o
m
i
c
e
v
a
l
u
a
t
i
o
n
s
e
t
c
.
B
i
l
l
P
a
y
m
e
n
t
R
a
i
s
i
n
g
o
f
F
i
n
a
n
c
e
p
o
p
u
l
a
t
i
o
n
a
t
r
i
s
k
A
n
a
l
y
s
i
s
o
f
P
r
e
v
e
n
t
i
o
n
P
r
i
m
a
r
y
c
a
r
e
S
e
c
o
n
d
a
r
y
&
T
e
r
t
i
a
r
y
c
a
r
e
L
o
n
g
-
t
e
r
m
c
a
r
e
o
f
f
i
n
a
n
c
e
R
a
i
s
i
n
g
R
e
s
e
a
r
c
h
D
e
v
e
l
o
p
m
e
n
t
&
D
i
s
t
r
i
b
u
t
i
o
n
M
a
n
u
f
a
c
t
u
r
i
n
g
&
S
a
l
e
s
M
a
r
k
e
t
i
n
g
Payer
Provider
Pharma
R
a
i
s
i
n
g
p
r
e
m
i
u
m
s
o
r
t
a
x
e
s
,
C
o
l
l
e
c
t
i
n
g
o
u
t
-
o
f
-
p
o
c
k
e
t
p
a
y
m
e
n
t
s
R
e
f
e
r
r
a
l
m
a
n
a
g
e
m
e
n
t
,
m
o
n
i
t
o
r
i
n
g
&
p
a
y
m
e
n
t
o
f
h
e
a
l
t
h
c
a
r
e
p
r
o
v
i
d
e
r
s
’
b
i
l
l
s
M
e
d
i
c
a
l
S
e
r
v
i
c
e
s
M
a
n
a
g
e
m
e
n
t
P
r
o
v
i
s
i
o
n
o
f
C
o
v
e
r
Figure 2: By 2020, the pharmaceutical, payer and provider value chains will be much more closely intertwined
Source: PricewaterhouseCoopers

6 PricewaterhouseCoopers
Choosing between
different collaborative
models
One vital question remains, however;
namely, what sort of model should
companies use to effect these changes?
We believe that two principal models
– federated and fully diversified – will
emerge. We have also identified two
variants of the federated model. In the
virtual version, a company outsources
most or all of its activities; in the
venture version, it manages a portfolio
of investments (see Figure 3). The two
models are not mutually exclusive. A
fully diversified company might choose
to use a federated model for certain
aspects of its business, and vice versa.
But we think that the federated model
will ultimately dominate, primarily
because it is quicker and more
economical to implement.
The federated model
In the federated approach, a company
creates a network of separate
entities with a common supporting
infrastructure. These might include
universities, hospitals, clinics,
technology suppliers, data analysis
firms and lifestyle service providers
based in numerous countries. They
might also include business units from
within the company itself, which it
places at “arm’s length” (see Figure 4).
The various participants have a mutual
goal – such as the management of
outcomes in a given patient population.
They also share funding, data, access
to patients and back-office services,
and this interdependence is the
glue that holds them together. They
are rewarded for their efforts using
measures like increased life expectancy
Virtual Variant
Venture Variant
Owned: Fully Diversified Model Collaborative: Federated Model
• Network of separate entities
• Based on shared goals & infrastructure
• Draws on in-house and/or external assets
• Combines size with flexibility
• Network of contractors
• Activities coordinated by one company
acting as hub
• Operates on project-by-project basis
• Fee-for-service financial structure
• Portfolio of investments
• Based on sharing of intellectual property/
capital growth
• Stimulates entrepreneurialism & innovation
• Spreads risk across portfolio
• Network of entities owned by one
parent company
• Based on provision of internally integrated
product-service mix
• Spreads risk across business units
Figure 3: The different business models
Source: PricewaterhouseCoopers

Pharma 2020:
Challenging business models
7
or quality-adjusted life years. And each
is rewarded in a manner that reflects
the evidence base for the contribution it
has made (see sidebar, How should the
cake be sliced?).
16
The federated model provides a
framework for creating integrated
packages of products and services, and
thus diversifying beyond a company’s
core offering. It also combines the
benefits of nimbleness and size. It
would enable each player to build a
specific area of expertise, establish a
competitive advantage as a result of
that expertise and sell its products,
knowledge or skills, leaving activities
that are better performed by others to
its partners within the federation.
More importantly still, the federated
model might encourage greater
cross-fertilisation and deliver bigger
improvements in performance, without
forfeiting any flexibility. The stronger
members of the network could help
the weaker ones to improve – since
federations have an incentive to perform
well as a whole – but they could also
replace any participant that persistently
underperforms.
Federation
T
e
c
h
n
o
l
o
g
y
G
e
n
e
r
i
c
s
C
o
m
p
l
i
a
n
c
e
M
a
n
a
g
e
m
e
n
t
C
e
n
t
r
e
s
W
e
i
g
h
t
F
i
t
n
e
s
s
C
l
u
b
s
C
o
m
p
a
n
y
U
n
i
v
e
r
s
i
t
i
e
s
C
l
i
n
i
n
c
s
H
o
s
p
i
t
a
l
s
P
h
y
s
i
o
t
h
e
r
a
p
y
A
n
a
l
y
s
t
s
D
a
t
a
S
u
p
p
l
i
e
r
s
C
a
l
l
C
e
n
t
r
e
M
a
n
u
f
a
c
t
u
r
e
r
s
P
h
a
r
m
a
c
e
u
t
i
c
a
l
C
e
n
t
r
e
s
Figure 4: The federated model
How should the cake be sliced?
It may sometimes be hard to measure
the value different participants have
created for two reasons. First, the
parties in any collaboration typically
value the contributions they have
made more highly than those of their
partners. This is a problem that can
be solved with watertight contracts,
robust performance indicators, good
governance and a proper audit trail.
Second, assessing the impact of
different forms of intervention can be
very difficult indeed.
Medicines, diet and exercise all play
a role in managing cardiovascular
disease, for example, but precisely how
much? Various studies have established
some parameters. They show, for
instance, that high-frequency exercise
can improve the cardio-respiratory
fitness of patients with heart disease
by at least 10% – and that, in turn,
can reduce the mortality rate by 15%.
We believe that many more studies
to evaluate the effectiveness of non-
pharmacological interventions will be
conducted in future, as healthcare
payers everywhere focus more heavily
on preventative measures.
This approach is essentially a more
complex variant of the co-development
and co-distribution agreements we have
today. In order for companies to work
better collaboratively it is essential to
define upfront measurable components
of delivery and value.
Defining the value provided by each
player in the federation will then inform
how each party should be rewarded -
this will be a combination of theoretical
analysis and monitoring of outcomes
and benefits to the patient. Clearly
to avoid the risk of litigation or the
constraints of exclusivity, the federation
needs to be underpinned by mutual
trust between all parties. However, there
are several examples of where this has
worked effectively such as a franchising
model where the value of a brand is
measured and rewarded.
Source: PricewaterhouseCoopers

Table of contents
8 PricewaterhouseCoopers
The virtual variant of the federated
model
In the virtual variant of the federated
model most or all of a company’s
operations are outsourced and the
company itself acts as a management
hub, coordinating the activities of
its partners (see Figure 5). Several
industries have already adopted
some aspects of this model. The
semiconductor industry typically
outsources its manufacturing in
order to concentrate on product
development, for example, and a
number of companies in the medical
devices sector are now following suit.
17
Similarly, strategic outsourcing of
design and manufacture to suppliers
has redefined manufacturing functions
within industries such as aerospace,
computing and electronics.
Most large pharmaceutical companies
also use external contractors to
supplement their in-house resources,
but very few firms have gone any
further (see sidebar, Shire’s virtual
vision).
18
There are very good reasons
why pharmaceutical companies should
outsource their R&D, manufacturing
and promotional activities where third
party alliances can provide a wider
range of opportunities, specialist
skills and market access. A pharma
company can then focus on the value
adding functions where they can
leverage on their relationships, scale
and market knowledge – i.e., project
management, business development,
regulatory affairs, intellectual property
management and the formation of good
relationships with key opinion leaders
Management
Hub
R
e
s
e
a
r
c
h
S
a
l
e
s
&
M
a
r
k
e
t
i
n
g
D
i
s
t
r
i
b
u
t
i
o
n
M
a
n
u
f
a
c
t
u
r
i
n
g
D
e
v
e
l
o
p
m
e
n
t
Figure 5: The virtual variant of the federated model
Source: PricewaterhouseCoopers
Shire’s virtual vision
Shire Pharmaceuticals is the epitome of a virtual company. It outsources almost
everything, from discovery to medical monitoring to data management to
statistics to medical writing. With the exception of its genetic therapy division,
every product it develops has been purchased from an outside source, via
in-licensing or acquisition.
Pharma 2020:
Challenging business models
9
and healthcare providers.
The virtual variant of the federated
model has other advantages, too. It
would enable companies to reduce
their initial capital outlay, convert
some of their fixed costs into variable
costs, utilise their resources more
efficiently and become more flexible.
Equally important, it might help the
industry leaders to expand into new
product/service areas or geographic
markets without resorting to further
mega-mergers (and thus facing the
huge challenges associated with
integrating two formerly separate
entities) or succumbing to the corporate
bureaucracy that so often strangles
innovation.
However, the virtual variant also comes
with some significant drawbacks.
The balance of power might shift to
suppliers, as it has done to a certain
extent in the automotive industry,
where a number of Tier 1 suppliers
now manage their own supply chains.
Alternatively, a major supplier might
get into financial difficulties and start
offering an inferior service or even
default on its obligations altogether.
But such risks can often be managed
by using multiple suppliers, wherever
possible.
Some pharmaceutical companies
might also see their earnings diluted,
since every participant in the value
chain would expect a return for the
services it provides. Theoretically, this
should not happen, since specialist
contractors typically have lower
costs than integrated pharmaceutical
companies. Indeed, according to one
study, a company that performs certain
preclinical development activities in-
house can expect to pay more than
double what it would pay if it completely
outsourced these activities to a third
party.
19
But a shortage of top-class
service providers or experts in particular
areas such as biological manufacturing
could drive prices up.
The venture variant of the
federated model
The venture variant of the federated
model entails investing in a portfolio of
companies in return for a share of the
intellectual assets and/or capital growth
they generate, rather than outsourcing
specific tasks. Special purpose vehicles
are sometimes used to manage such
investments, because they offer several
advantages in terms of risk sharing and
intellectual property protection.
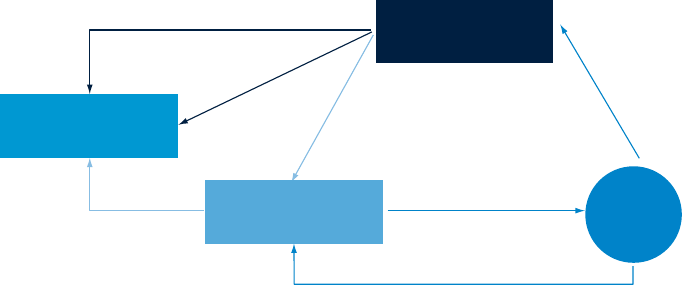
A pharmaceutical company might
choose to concentrate its investments
in a particular therapeutic area or
spread them across a number of areas
in order to minimise its risk. At the end
of the investment period, it might either
claim the intellectual property that has
been generated or out-license it to a
third party. Alternatively, the originating
company (or companies) might retain
the intellectual property, commercialise
it and pay the sponsoring company a
return on its investment (see Figure 6).
GlaxoSmithKline has used a version
of the venture structure for many
years. SR One, its evergreen fund,
was established in 1985 and has
now invested more than US$500m in
some 30 private and public biotech
companies focusing on drug discovery,
development and delivery.
20
Other
Big Pharma companies, such as
Novartis and Pfizer, have also set up
corporate venture capital funds,
21
and AstraZeneca spun off part of its
gastrointestinal research operation
into a new company backed by a
consortium of private equity firms.
22
US investment bank Goldman Sachs
Generation of IP
Return of IP to growth company
Out-licensing/In-licensing
Out-licensing
Royalty payment
Investment
Return of IP to
pharmaceutical company
Pharmaceutical
company
Third party
IP
Growth
company
Figure 6: The venture variant of the federated model
Source: PricewaterhouseCoopers

10 PricewaterhouseCoopers
has already dipped a toe in the water
with its own venture fund (see sidebar,
Portfolio of pills).
23
Nevertheless, all these initiatives
are very small; between 2003 and
September 2006, corporate venture
capitalists invested just over US$1.5
billion in the US life sciences sector,
24
a fraction of the estimated US$11-
15 billion the member companies of
the Pharmaceutical Research and
Manufacturers of America spent on
discovery in 2006 alone.
25
Most such
ventures are also confined to research,
although the same approach could be
applied to development, manufacturing,
distribution, and marketing and sales.
So what might the venture variant
deliver, if it were implemented on a
much larger scale and extended to
other parts of the value chain? It would
alleviate the funding challenges in the
biotech sector, where companies often
struggle to raise a second or third
round of financing because venture
capitalists want to exit before they can
commercialise their products. These
challenges have been exacerbated by
the credit crunch and are likely to get
even worse in the current economic
recession.
26
It would also allow
promising start-ups to capitalise on
Big Pharma’s experience without being
stifled by a Big Pharma culture – both
trends which might stimulate greater
innovation.
Similarly, it would provide incentives for
traditional contract service providers to
make strategic, long-term investments –
as Lonza did, when it collaborated with
Genentech to build a manufacturing
plant in Singapore.
27
And it would
enable pharmaceutical companies to
explore numerous new avenues of R&D,
or expand their global manufacturing
and marketing capacity, without
investing too heavily in any one project.
However, venture structures are not
without their challenges. For a start,
the skills involved in managing a
portfolio of holdings are very different
from those involved in assessing and
pursuing potential research leads, as
is the timeframe venture capitalists
use to realise a return. So Big Pharma
would need to recruit people with the
necessary expertise and manage any
conflicting objectives very carefully.
Moreover, any company that operated
a large corporate venture capital fund
alongside its own research portfolio
would have to consider the financial
implications very carefully. R&D
expenditure is typically recorded on a
company’s profit and loss statement,
for example, whereas investments are
registered on the balance sheet and
subject to annual impairment reviews.
This has an impact on how companies
are taxed and on how they are valued
by the stock markets. Similarly, if
a company’s risk profile increases
because it has less control over research
that is conducted outside its own walls,
its cost of capital will increase.
Goldman Sachs has funded a
new “research pool” into which
pharmaceutical companies could
place a range of experimental
medicines in a single therapeutic area
in early-stage Phase I and II trials.
External experts, including scientists,
chemists and clinical research
organisations, would work alongside
scientists from the originating
companies. The bank argues that this
approach would reduce the costs
and bureaucracy associated with Big
Pharma. It might also allow competing
companies working on similar drugs
to pool their resources, rather than
duplicating each other’s efforts.
In April 2009, GSK and Pfizer
announced that they intend to
combine resources to set up a new
spin off firm dedicated to the HIV.
The structure of the deal gives a
majority 85% stake to GSK and 15%
to Pfizer with an increase of Pfizer’s
stake to 24.5% if all milestones are
reached. The new firm, with a current
revenue of £1.6 billion has a portfolio
of 11 products and a drug-discovery
pipeline of 17. R&D services will be
contracted directly from GSK and
Pfizer to develop these drugs with
investment from the new firm. In
return, the new firm will have exclusive
rights of first negotiation with respect
to HIV drugs developed by the two
pharma majors. The rationale for the
venture is that the new firm will be
more sustainable and broader as a
combined venture and that there are
synergies on the commercial side.
Portfolio of pills

Pharma 2020:
Challenging business models
11
The fully diversified model
The fully diversified model is one in
which a company expands from its
core business into the provision of
related products and services, such
as diagnostics and devices, generics,
nutraceuticals and health management
(see Figure 7). Johnson & Johnson
is Pharma’s leading exponent of this
approach. It is now the world’s largest
consumer health company, following
the US$16.6 billion acquisition of
Pfizer’s over-the-counter business
in December 2006.
28
It is also the
third-largest biologics and sixth-
largest pharmaceutical company, has
an extensive medical devices and
diagnostics operation,
29
and recently
started building a wellness and
prevention platform, with the purchase
of HealthMedia, a web-based “health
coach”.
30
A number of other companies are now
following suit. Novartis has spent nearly
US$25 billion beefing up its vaccines,
generics and eye-care products
operations over the past three years,
for example.
31
Roche is drawing on
its expertise in molecular diagnostics
to develop a consumer product test
for measuring indoor allergens.
32
And
GlaxoSmithKline has announced plans
to “diversify and de-risk” by focusing
more heavily on vaccines, consumer
health and the emerging markets.
33
The fully diversified model has several
merits, not least the fact that it enables
companies to reduce their reliance on
blockbuster medicines and spread their
risk by moving into other market spaces
with the potential to act as a bulwark
against generic competition. Like
the federated model, it also provides
a means of moving into outcomes
management by offering combined
product-service packages and playing
to the growing political emphasis on
prevention rather than treatment.
In addition to these advantages, it might
offer opportunities both to develop more
powerful brands and to acquire a better
corporate image. Numerous studies
show the extent to which Pharma’s
reputation has declined over the past
decade.
34
Supplementing its products with
“wellness” services might help a company
to create a more positive impression,
although it would have to handle its
relations with the regulators, healthcare
providers and patients very carefully.
Ethical
Pharmaceuticals
Diagnostics
& Devices
Generics
Consumer
Health
Health
Management
• Molecular testing
• Clinical biomarkers
• Medical devices
• Branded generics
• Commodity generics
• Super-generics
• Follow-on biologicals
• Over-the-counter
medicines
• Consumer
diagnostics
• Nutraceuticals
Mass-Market
• Primary-care
products (including
patches, inhalants
and controlled-release
implants)
• Poly-pills
Specialised-Market
• Biologicals
• Orphan drugs
• Vaccines
• Patient education
• Delivery and drug
administration
services
• Monitoring and
counselling
• Physiotherapy
• Nutritional advice
• Wellness
management
Source: PricewaterhouseCoopers
Figure 7: The fully diversified model

Table of contents
12 PricewaterhouseCoopers
However, the fully diversified model has
drawbacks, too. It requires a substantial
investment in new equipment, premises
and personnel, as well as major cultural
changes, since the provision of products
is very different from the provision of
services. It might also create new risks
by distracting management’s attention
from the core business – and even
alienate investors, who often prefer to
spread risk themselves.
Charting a successful
course
Clearly, the business model, or models,
a company chooses will depend on
its individual circumstances, including
the particular challenges it faces, the
expertise it possesses and the markets
in which it wants to operate. A company
that focuses exclusively on ethical
pharmaceuticals might find it harder
to diversify than one that is already
experienced in managing multiple
areas of activity, for example. Moreover,
federations typically place greater
demands on senior management than
conventional organisational hierarchies.
Creating and supervising a cross-
border, cross-disciplinary network
of external relationships can be very
time-consuming – and it is often
more difficult to identify, monitor and
manage risks. The various parties may
have different cultural characteristics,
different ways of communicating and
different expectations, some of which
may change over time. An individual
manager’s authority over the other
participants in the network is also likely
to be relatively limited. In a heavily
regulated industry such as Pharma, any
diminution of managerial control has
serious implications. So it is crucial to
establish clear goals and guidelines for
the governance and funding of such
arrangements, and for the division of
any intellectual assets they generate,
before signing on the dotted line.
Disrupting the existing order can
have a major impact on a company’s
short-term performance, too. When
GlaxoSmithKline established its Centres
of Excellence for Drug Discovery,
the upheavals the R&D function was
experiencing affected its pipeline for at
least 18 months.
35
We think that many companies which
choose the federated model will
therefore adopt a progressive approach.
They will start with opportunistic
alliances; use the most successful
alliances as building blocks to create
more strategic, longer-lasting coalitions;
and, finally, use the most successful
coalitions to create a fully federated
network of long-term partners (see
Figure 8). Taking incremental steps
will not only help them to identify the
organisations with which they can work
most effectively, but also give them
time to establish the technological
infrastructure that is essential to
manage the interfaces between two or
more different parties.
Most companies will also have to recruit
or train people with new skills. They will,
for example, need researchers who can
understand commercial imperatives;
financial analysts who can assess
different investment opportunities with
the discipline of venture capitalists;
senior executives who can negotiate
and oversee alliances; supply chain
managers who can supervise large
networks of service providers; and
health economists who can measure the
value of the contributions the respective
parties make. Those that choose to enter
the health management space directly
will also have to hire physiotherapists,
Opportunistic
Alliances
Strategic
Coalitions
Full
Federation
• Extended alliances
• Medium-term
• Three or more parties
• One-to-many
relationship
• Closer alignment
• Extended coalitions
• Long-term
• Many parties
• Many-to-many
relationship
• Complete alignment
• Ad-hoc
• Short-term
• Two parties
• One-to-one relationship
• Partial alignment
Figure 8: The path to federation is likely to be gradual
Source: PricewaterhouseCoopers

Pharma 2020:
Challenging business models
13
dieticians, counsellors and numerous
other people with skills that were
formerly outside Pharma’s domain.
Finding people with the appropriate
expertise will not be easy. Many
companies will therefore have to adopt
new talent management strategies, as
well as ensuring that the performance
measures and incentive systems they
use support the behaviour they want to
encourage.
Conclusion
Pharma’s fully integrated business
model enabled it to profit alone
for many years – and to profit very
successfully, as its track record in
rewarding shareholders shows. The
top companies saw their market
value soar 85-fold between 1985 and
2000.
36
But this model is now under
huge pressure and, by 2020, it will
not work. If the industry is to improve
its performance in the lab, reduce its
costs, serve the emerging markets
more effectively and make the transition
from producing medicines to managing
outcomes – as healthcare payers,
providers and patients are increasingly
demanding – it will have to collaborate
with other organisations, both inside
and outside the sector. It simply cannot
do everything itself. In addition there is
a clear economic rationale for greater
collaboration (See sidebar, Show me
the money).
Moreover, many companies will need to
move fast. As the healthcare landscape
changes and scientific expertise
becomes less important than the ability
to manage networks, the scope for
competition from new entrants will
increase. Several non-pharmaceutical
companies have already entered the
arena. Vodafone has, for example,
joined forces with Spanish telemedicine
provider Medicronic Salud and device
manufacturer Aerotel Medical Systems
to offer a wireless home monitoring
service.
37
Similarly, British insurance
Show me the money
There is plenty of evidence pointing to big opportunities for savings to be made through early intervention and tighter
management of patients and treatments. The federated model will make these savings more systematic and predictable,
rewarding participants based on the value that they create. Aligning risk and incentives appropriately is key to realising these
benefits. For example:
A study by the RAND Corporation estimated the financial savings from having 100% participation in disease management •
programmes for four diseases (asthma, chronic obstructive pulmonary disease, diabetes and congestive heart failure) in
the US. They estimate the net savings to the health system to be $28bn (around 2% of total US health expenditure), with
additional benefits to the economy in terms of work days saved.
38
Britain’s Audit Commission examined the scale of adverse events in UK hospitals. They found that 10.8% of patients on •
medical wards experience an adverse event, 46% of which are preventable. One third of the adverse events lead to greater
morbidity or death and cost the UK’s NHS £1.1bn a year.
39
The five most costly conditions collectively account for 32.7% of overall healthcare expenditure. As we highlighted in •
“Pharma 2020: The vision”, improving patient compliance with enhanced treatment regimes by collaborating with other
support services is a key enabler to drive the healthcare bill down. Further, some commentators have suggested giving
patients financial incentives to improve compliance.
40
In 2009, Cisco Systems reported healthcare cost savings of $2.6m from a programme of on-site medical clinics covering 6,000 •
employees supported by integrated healthcare technology systems, chronic disease management, and health coaching.
41

14 PricewaterhouseCoopers
giant Prudential is collaborating with
Virgin Active Health Club to offer a
critical illness policy that provides
subsidised gym membership and
rewards people who exercise regularly
by reducing their premiums.
42
If the
leading pharmaceutical companies
cannot change their business models
rapidly, such firms may ultimately
feature more prominently on the
healthcare scene than they themselves.
The transition will not be easy, for
collaborative business models are
far more complex than the integrated
model that has previously prevailed.
Moreover, no one model will suit every
company. Each will need to assess
its position, options and future course
in light of its individual strengths and
needs (see sidebar, Key questions for
senior management).
However, the prospects for any
pharmaceutical company that can make
the switch are very promising. The
potential for reallocating resources to
deliver better outcomes and maximise
the effectiveness of expenditure
on healthcare is considerable in
most healthcare systems. Research
recently completed by Britain’s Audit
Commission shows, for example, that
annual spending on the treatment of
diabetes ranges from less than £8 to
over £30 (US$11.9-US$44.6) per head.
43
But differences in the prevalence of
diabetes account for only 8% of this
variation – and higher expenditure does
not result in fewer emergency hospital
admissions.
44
To date, Pharma has focused on the
profits it can earn from the estimated
10-15% of the health budget that goes
on medicines.
45
Yet there are many
opportunities to generate revenues
by improving the way on which the
remaining 85-90% is spent. It is these
opportunities the industry will need to
address in the brave new world of 2020.
Key questions for senior
management
What is our current business •
model? Does it play sufficiently to
our strengths?
What kind of company do we •
want our company to be?
Will our current business model •
enable us to expand into
new markets – be these new
products, services or countries
– and satisfy the expectations of
our customers in 2020? If not,
what sort of business model will
we need?
What is the size of the gap and •
how can we reduce it as rapidly
as possible?
Do we have a clear picture of the •
opportunities and risks entailed
by each of the alternatives
available to us?
Do we have a plan in place that •
will enable us to move forward
quickly, while maximising the
opportunities and minimising the
risks?
Pharma 2020:
Challenging business models
15
We would like to thank the many people at PricewaterhouseCoopers who helped us to develop this report. We would also like
to express our appreciation for the input we received from clients and our particular gratitude to the following external experts
who so generously donated their time and effort to the project;
Adrian Rawcliffe, Senior Vice President Worldwide Business Development, GlaxoSmithKline Plc.
Dr Dennis B Gillings, Chairman of the Board and Founder, Quintiles Transnational Corp.
Dr Genghis Lloyd-Harris, Partner, Abingworth
Gino Santini, Senior Vice President, Corporate Strategy and Policy, Eli Lilly and Co.
John Fowler, Head of Healthcare Investment Banking Team in Europe, Deustche Bank AG
Dr John Murphy, European Pharmaceuticals Analyst, Goldman Sachs Group, Inc.
Laurent Massuyeau, Head of Business Development, Addex Pharmaceuticals Ltd.
Professor Michael Jacobides, London Business School
Dr Vincent Mutel, Chief Executive Officer, Addex Pharmaceuticals Ltd.
The views expressed herein are personal and do not reflect the views of the organisations represented by the individuals
concerned.
Acknowledgements

Table of contents
Pharma 2020:
Challenging business models
17
PricewaterhouseCoopers, “Pharma 2020: The vision – Which path will you take?” (June 2007). 1.
Kathryn Phelps, “Mergers May Buy Time, But Fundamental Changes Necessary, GSK’s Garnier”, 2. The Pink Sheet (February 26, 2007), p. 11.
Clayton M. Christensen, “The Innovator’s Dilemma: When New Technologies Cause Great Firms to Fail” (Boston: Harvard Business Press, 1997).3.
Michael G. Jacobides, Thorbjørn Knudsen & Mie Augier, “Who Does What and Who gets What: Capturing the Value from Innovation”, AIM 4.
Executive Briefing (2006), accessed July 27, 2009, http://faculty.london.edu/mjacobides/assets/documents/JKAbriefingAIMnov06.pdf
“Rhône-Poulenc Forms Network To Develop Gene and Cell Therapies”,5. Oncology News International, Vol. 4, No. 1 (January 1, 1995), accessed
December 8, 2008, http://www.cancernetwork.com/display/article/10165/97578
The Boston Consulting Group, “Realizing the Promise of Disease Management: Payer Trends and Opportunities in the United States” (February 6.
2006), p. 9.
Michael G. Jacobides, Thorbjørn Knudsen & Mie Augier, “Benefiting from Innovation: Value Creation, Value Appropriation and the Role of Industry 7.
Architectures”, Research Policy, Vol. 35 (2006): 1200-1221.
BBC News, “Deal reached on NHS drug prices” (November 19, 2008), accessed November 28, 2008, http://news.bbc.co.uk/1/hi/health/7737027.stm8.
“Barack Obama and Joe Biden’s plan to lower health care costs and ensure affordable, accessible health coverage for all” (2008), accessed 9.
November 10, 2008, http://www.barackobama.com/pdf/issues/HealthCareFullPlan.pdf
James J. Shen, “Will China’s healthcare reform save the day?” 10. Pharma China, Issue 27 (November 2008), pp. 2-5.
OECD, “OECD Science, Technology and Industry Outlook 2008: Highlights” (2008), accessed December 22, 2008, http://www.oecd.org/11.
dataoecd/18/32/41551978.pdf
PricewaterhouseCoopers “Pharma 2020: Virtual R&D – Which path will you take?” (June 2008)12.
For an extensive discussion of these trends, please see “Pharma 2020: Virtual R&D”.13.
Lilly website, accessed December 8, 2008, http://www.lilly.com/about/partnerships/; and Debiopharm Group website, accessed December 8, 14.
2008, http://www.debiopharm.com/business-model.html
PricewaterhouseCoopers, “Pharma 2020: Marketing the future – Which path will you take?” (February 2009)15.
University of Florida Health Science Center media release, “Walk this way: UF research provides insight into heart healthy exercise regimen” 16.
(November 14, 2005), http://news.health.ufl.edu/news/story.aspx?ID=2846
David Busch, “Can History Repeat Itself? The Case for the Virtual Medical Device OEM”, 17. Medical Product Outsourcing (December 2007),
accessed February 21, 2008, http://www.mpo-mag.com/articles/2007/11/can-history-repeat-itself
Kenneth Eng, David C. Izard & Terek J. Peterson, “Managing the CRO Relationship to Effectively Request and Receive CDISC STDM and ADaM 18.
Deliverables”, SAS PharmaSUG Proceedings (Atlanta, Georgia: June1-4, 2008), accessed December 12, 2008, http://www.lexjansen.com/
pharmasug/2007/fc/fc06.pdf
SRI International, “Capital and Time Efficiencies from Outsourcing Preclinical Development” (2005), accessed March 10, 2008, http://www.sri.19.
com/biosciences/pdf/OutsourcingPrecelinicalDevelopment.pdf
SR One website, accessed December 14, 2008, http://www.srone.com/20.
Lisa M. Jarvis, “Thinking Outside The Big Pharma Box”, 21. Chemical & Engineering News (May 7, 2007), accessed February 20, 2008, http://
pubs.acs.org/cen/coverstory/85/8519cover2.html; and Associated Press, “Pfizer expands venture capital program” (August 1, 2007), accessed
February 20, 2008, http://www.boston.com/business/articles/2007/08/01/pfizer_expands_venture_capital_program/
Jonathan Russell, “AstraZeneca spins off research”, 22. The Telegraph (February 15, 2008), accessed March 9, 2008, http://www.telegraph.co.uk/
money/main.jhtml?xml=/money/2008/02/15/cnastra115.xml
Andrew Jack, “Goldman unveils plans for pharma research funding”, The 23. Financial Times (December 3, 2008), accessed December 12, 2008,
http://www.ft.com/cms/s/0/e92f9d3c-c0db-11dd-b0a8-000077b07658.html?nclick_check=1
PricewaterhouseCoopers/National Venture Capital Association MoneyTree Report based on data from Thomson Financial. 24.
The member companies of the Pharmaceutical Research and Manufacturers of America spent about US$43 billion on R&D in 2006. Datamonitor 25.
estimates that discovery accounts for between 25% and 35% of these costs. For further information, see Pharmaceutical Research and
Manufacturers of America (PhRMA), “R&D Spending by U.S. Biopharmaceutical Companies Reaches a Record $55.2 Billion in 2006” (February
12, 2007), accessed February 20, 2008, http://www.phrma.org/news_room/press_releases/r&d_spending_by_u.s._biopharmaceutical_companies_
reaches_a_record_$55.2_billion_in_2006/; and Datamonitor, “Pharmaceutical Outsourcing Part 2: An Introduction to Drug Discovery Strategies”
(August 2006).
References
18 PricewaterhouseCoopers
Andrew Jack, “Drugmakers sweeten deals for biotech groups”, The 26. Financial Times (September 19 2008), accessed December 21, 2008, http://
www.ft.com/cms/s/0/9d620a56-85d6-11dd-a1ac-0000779fd18c,dwp_uuid=aece9792-aa13-11da-96ea-0000779e2340.html
Jim Miller, “The Art of the Deal”, 27. BioPharm International (September 1, 2008), accessed December 10, 2008, http://biopharminternational.
findpharma.com/biopharm/GMPs%2FValidation/The-Art-of-the-Deal/ArticleStandard/Article/detail/545356
Andrew Ross Sorkin & Stephanie Saul, “J&J buys Pfizer unit for $16.6 billion”, 28. International Herald Tribune (June 26, 2006), accessed November 3,
2007, http://www.iht.com/articles/2006/06/26/business/pfizer.php
Johnson & Johnson website, accessed December 11, 2008, www.jnj.com/connect/about-jnj29.
Johnson & Johnson press release, “Johnson & Johnson Establishes Wellness & Prevention Platform with Acquisition of HealthMedia, Inc.” 30.
(October 27, 2008), accessed December 11, 2008, www.jnj.com/connect/news/corporate/20081027_151000
Sarah Rubenstein, “Novartis Spends Big on Diversification”, The Wall Street Journal Health Blog (April 7, 2008), accessed December 21, 2008, 31.
http://blogs.wsj.com/health/2008/04/07/novartis-spends-big-on-diversification/
Reuters, “National Jewish Health and Roche Diagnostics Corporation Reach Agreement to Pursue Environmental Molecular Diagnostics 32.
Opportunities” (September 10, 2008), accessed December 11, 2008, http://www.reuters.com/article/pressRelease/idUS134552+10-Sep-
2008+PRN20080910
Andrew Jack, “GSK chief pushes for expansion into new markets”, The 33. Financial Times (June 13, 2008), accessed December 21, 2008, http://
www.ft.com/cms/s/0/617a6ef8-38e1-11dd-8aed-0000779fd2ac.html?nclick_check=1
PricewaterhouseCoopers, “Pharma 2020: The vision”, pp. 24-25.34.
Robert S. Huckman & Eli P. Strick, “GlaxoSmithKline: Reorganizing Drug Discovery (B)”, 35. Harvard Business Publishing (May 17, 2005). Available for
order at http://harvardbusinessonline.hbsp.harvard.edu/b01/en/common/item_detail.jhtml?id=605075
Milken Institute, “Financial innovations for Accelerating Medical Solutions”, Vol. 2 (October 2006), accessed December 15, 2008, http://www.36.
milkeninstitute.org/pdf/fin_innov_vol2.pdf
Aerotel Medical Systems media release, “Aerotel and Medicronic-Vodafone Launch Innovative Wireless Homecare System in Spain” (April 4, 37.
2008), accessed December 8, 2008, http://www.openpr.com/news/41301/Aerotel-and-Medicronic-Vodafone-Launch-Innovative-Wireless-
Homecare-System-in-Spain.html
Bigelow JH et al “Analysis of healthcare interventions that change patient trajectories”, 38. The RAND Corporation, 2005, pg xxvi
Audit Commission “A spoonful of sugar: Medicines management in NHS hospitals”, December 2001, pg 1939.
For example, Giuffrida A and Torgerson DJ, “Should we pay the patient? Review of financial incentives to enhance patient compliance”. 40. British
Medical Journal 1997 315: 703-707
Cathy Weselby, “Improving health at the office”,41. Silicon Valley/San Jose Business Journal, accessed January 12, 2009, http://sanjose.bizjournals.
com/sanjose/stories/2009/01/12/story2.html
Helen Loveless, “The comeback kid’s medical cover”, 42. Mail on Sunday (October 29, 2007), accessed October 15, 2008, http://www.thisismoney.
co.uk/insurance/health-insurance/article.html?in_article_id=425744&in_page_id=39
Based on the midmarket exchange rate of £1.00 to US$1.48474 on December 22, 2008.43.
Audit Commission, “Health Data Briefing No. 12. Spending on disease: Diabetes” (September 2008), accessed December 15, 2008, http://www.44.
audit-commission.gov.uk/Health/Downloads/HealthDataBriefing_spendingondiabetes.pdf
Total expenditure on pharmaceuticals and other medical non-durables expressed as a percentage of total healthcare expenditure ranges from 45.
12.4% to 29.7% in the OECD countries. On average, it is thought to represent about 15% of the global health budget. For further information on
healthcare expenditure in the OECD countries, see OECD Health Data 2008 (October 2008).

Territory contacts
Argentina
Diego Niebuhr
[54] 11 4850 4705
Australia
John Cannings
[61] 2 826 66410
Belgium
Thierry Vanwelkenhuyzen
[32] 2 710 7422
Brazil (SOACAT)
Luis Madasi
[55] 11 3674 1520
Canada
Gord Jans
[1] 905 897 4527
Czech Republic
Radmila Fortova
[420] 2 5115 2521
Denmark
Torben TOJ Jensen
[45] 3 945 9243
Erik Todbjerg
[45] 3 945 9433
Finland
Janne Rajalahti
[358] 3 3138 8016
Johan Kronberg
[358] 9 2280 1253
France
Jacques Denizeau
[33] 1 56 57 10 55
Germany
Volker Booten
[49] 89 5790 6347
India
Sharat Bansal
[91] 22 6669 1538
Ireland
John M Kelly
[353] 1 792 6307
Enda McDonagh
[353] 1 792 8728
Israel
Assaf Shemer
[972] 3 795 4681
Italy
Massimo Dal Lago
[39] 045 8002561
Japan
Kenichiro Abe
[81] 80 3158 5929
Luxembourg
Laurent Probst
[352] 0 494 848 2522
Mexico
Ruben Guerra
[52] 55 5263 6051
Netherlands
Arwin van der Linden
[31] 20 5684712
Poland
Mariusz Ignatowicz
[48] 22 523 4795
Portugal
Ana Lopes
[351] 213 599 159
Russia
Alina Lavrentieva
[7] 495 967 6250
Singapore
Abhijit Ghosh
[65] 6236 3888
South Africa
Denis von Hoesslin
[27] 117 974 285
Spain
Rafael Rodríguez Alonso
[34] 91 568 4287
Sweden
Liselott Stenudd
[46] 8 555 33 405
Switzerland
Clive Bellingham
[41] 58 792 2822
Peter Kartscher
[41] 58 792 5630
Markus Prinzen
[41] 58 792 5310
Turkey
Ediz Gunsel
[90] 212 326 6060
United Kingdom
Andy Kemp
[44] 20 7804 4408

Table of contents
This publication has been prepared for general guidance on matters of interest only, and does not constitute professional advice. You should not act upon the information contained in this publication
without obtaining specific professional advice. No representation or warranty (express or implied) is given as to the accuracy or completeness of the information contained in this publication, and, to the
extent permitted by law, PricewaterhouseCoopers does not accept or assume any liability, responsibility or duty of care for any consequences of you or anyone else acting, or refraining to act, in reliance
on the information contained in this publication or for any decision based on it.
© 2009 PricewaterhouseCoopers. All rights reserved. ‘PricewaterhouseCoopers’ refers to the network of member firms of PricewaterhouseCoopers International Limited, each of which is a separate and
independent legal entity.
For further information, please contact:
pwc.com/pharma
Global
Simon Friend
Partner, Global Pharmaceutical and Life Sciences
Industry Leader
PricewaterhouseCoopers (UK)
[44] 20 7213 4875
Steve Arlington
Partner, Global Pharmaceutical and Life Sciences Advisory
Services Leader
PricewaterhouseCoopers (UK)
[44] 20 7804 3997
Michael Swanick
Partner, Global Pharmaceutical and Life Sciences Tax Leader
PricewaterhouseCoopers (US)
[1] 267 330 6060
United States
Anthony Farino
Partner, US Pharmaceutical and Life Sciences Advisory
Services Leader
PricewaterhouseCoopers (US)
anthony[email protected]
[1] 312 298 2631
Mark Simon
Partner, US Pharmaceutical and Life Sciences Industry Leader
PricewaterhouseCoopers (US)
[1] 973 236 5410
Middle East
Sally Jeffery
Partner, Healthcare Advisory Services, Middle East
PricewaterhouseCoopers (United Arab Emirates)
sally[email protected]
[971] 4 304 3154
Europe
Jo Pisani
Partner, Pharmaceuticals and Life Sciences, Strategy
PricewaterhouseCoopers (UK)
[44] 20 7804 3744
Sandy Johnston
Partner, European Pharmaceutical and Life Sciences
Advisory Services
PricewaterhouseCoopers (UK)
sandy[email protected]
[44] 20 7213 1952
Yann Bonduelle
Partner, Performance Improvement Consulting
PricewaterhouseCoopers (UK)
[44] 20 7804 5935
Asia Pacific
Sujay Shetty
Associate Director, Pharmaceutical and Life Sciences
Advisory Services, India
PricewaterhouseCoopers (India)
sujay[email protected]
[91] 22 6669 1305
Beatrijs Van Liedekerke
Associate Director, Pharmaceutical and Life Sciences
Advisory Services, China
PricewaterhouseCoopers (China)
[86] 10 6533 7223
Marketing
Attila Karacsony
Director, Global Pharmaceutical and Life Sciences
PricewaterhouseCoopers (US)
[1] 973 236 5640
Marina Bello Valcarce
Global Pharmaceutical and Life Sciences
PricewaterhouseCoopers (UK)
[44] 20 7212 8642
