
INSTRUCTIONS FOR USE
DUPIXENT
®
(DU-pix-ent)
(dupilumab)
injection, for subcutaneous use
Single-Dose Pre-filled Syringe with Needle Shield
Read this Instructions for Use before using the DUPIXENT Pre-filled Syringe. Do not inject the
child until you have been shown how to inject DUPIXENT. Your healthcare provider can
show you how to prepare and inject a dose of DUPIXENT before you try to do it yourself the first
time. Keep these instructions for future use. Call your healthcare provider if you have any
questions.
This device is a Single-Dose Pre-filled Syringe (called “DUPIXENT Syringe” in these
instructions). It contains 100 mg of DUPIXENT for injection under the skin (subcutaneous
injection).
The parts of the DUPIXENT Syringe are shown below:
Important Information
• Read all of the instructions carefully
before using the DUPIXENT Syringe.
• Ask your healthcare provider how often
you will need to inject the medicine.
• In children 6 months to less than 12
years of age, DUPIXENT should be
given by a caregiver.
• Rotate the injection site each time you
inject.
• Do not use the DUPIXENT Syringe if it
has been dropped on a hard surface or
damaged.
• To reduce the risk of accidental needle
sticks, each pre-filled syringe has a
Needle Shield that is automatically
activated to cover the needle after you
have given the injection.
• Do not pull back on the Plunger Rod at
any time.
• Do not remove the Needle Cap until just
before you give the injection.
• Throw away (dispose of) the used
DUPIXENT Single-Dose Pre-filled
Syringe right away after use. See “Step
13: Dispose” below.
Reference ID: 5208898
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

• Do not use the DUPIXENT Syringe if the
Needle Cap is missing or not securely
attached.
• Do not touch the Plunger Rod until you
are ready to inject.
• Do not inject through clothes.
• Do not get rid of any air bubble in the
DUPIXENT Syringe.
• Do not re-use a DUPIXENT Single-
Dose Pre-filled Syringe.
How should I store DUPIXENT?
• Keep DUPIXENT Syringes and all medicines out of the reach of children.
• Store DUPIXENT Syringes in the refrigerator between 36°F to 46°F (2°C to 8°C).
• Store DUPIXENT Syringes in the original carton to protect them from light.
• DUPIXENT Syringes can be stored at room temperature up to 77°F (25°C) up to 14 days.
Throw away (dispose of) any DUPIXENT Syringes that have been left at room
temperature for longer than 14 days.
• Do not shake the DUPIXENT Syringe.
• Do not heat the DUPIXENT Syringe.
• Do not freeze the DUPIXENT Syringe.
• Do not put the DUPIXENT Syringe into direct sunlight.
Step 1: Remove
Remove the DUPIXENT Syringe from the carton by holding the middle of the Syringe Body.
Do not pull off the Needle Cap until you are ready to inject.
Do not use the DUPIXENT Syringe if it has been dropped on a hard surface or
damaged.
Step 2: Prepare
Ensure you have the following:
• the DUPIXENT Pre-filled Syringe
• 1 alcohol wipe*
• 1 cotton ball or gauze*
Reference ID: 5208898
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

• a sharps disposal container* (See Step 13)
*Items not included in the carton
Step 3: Check
When you receive the DUPIXENT Syringes, always check to see that:
• you have the correct medicine and dose.
• the expiration date on the Single-Dose Pre-filled Syringe has not passed.
Do not use the DUPIXENT Syringe if the expiration date has passed.
Step 4: Inspect
Look at the medicine through the Viewing Window on the DUPIXENT Syringe:
Check to see if the liquid is clear and colorless to pale yellow.
Note: You may see an air bubble, this is normal.
Do not use the DUPIXENT Syringe if the liquid is discolored or cloudy, or if it contains
visible flakes or particles.
Reference ID: 5208898
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Step 5: Wait 30 minutes
Lay the DUPIXENT Syringe on a flat surface and let it naturally warm to room temperature for at
least 30 minutes.
Do not heat the DUPIXENT Syringe.
Do not put the DUPIXENT Syringe into direct sunlight.
Do not keep DUPIXENT Syringes at room temperature for more than 14 days. Throw
away (dispose of) any DUPIXENT Syringes that have been left at room temperature
for longer than 14 days.
Step 6: Choose the injection site
• You can inject into the thigh, outer area of the upper arm or stomach, except for the 2
inches (5 cm) around the belly button (navel).
• Choose a different site each time you inject DUPIXENT.
Do not inject into skin that is tender, damaged, bruised or scarred.
Reference ID: 5208898
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Step 7: Clean
Wash your hands.
Clean the injection site with an alcohol wipe.
Let the skin dry before injecting.
Do not touch the injection site again or blow on it before the injection.
Step 8: Remove Needle Cap
Hold the DUPIXENT Syringe in the middle of the Syringe Body with the Needle pointing away
from you and pull off the Needle Cap.
Do not put the Needle Cap back on.
Reference ID: 5208898
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
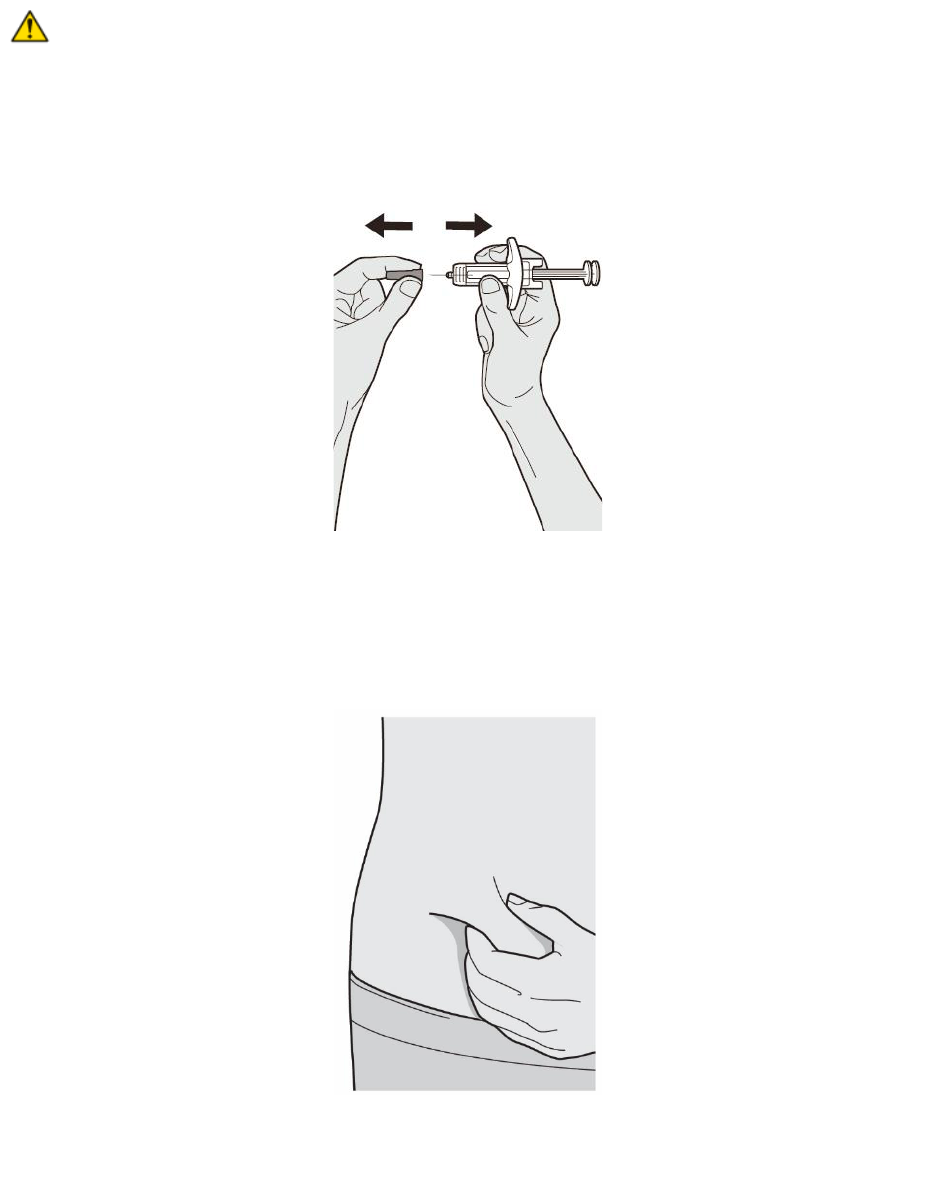
Do not touch the Needle.
Inject the medicine right away after removing the Needle Cap.
Step 9: Pinch
Pinch a fold of skin at the injection site (thigh or stomach, except 2 inches around the belly
button, or outer area of the upper arm). The figure below shows an example of pinching a fold of
skin on the stomach.
Step 10: Insert
Insert the Needle completely into the fold of the skin at about a 45° angle.
Reference ID: 5208898
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Step 11: Push
Relax the pinch.
Push the Plunger Rod down slowly and steadily as far as it will go until the DUPIXENT Syringe
is empty.
Note: You will feel some resistance. This is normal.
Step 12: Release and Remove
Lift your thumb to release the Plunger Rod until the Needle is covered by the Needle Shield and
then remove the Syringe from the injection site.
Lightly press a cotton ball or gauze on the injection site if you see any blood.
Do not put the Needle Cap back on.
Do not rub the skin after the injection.
Reference ID: 5208898
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

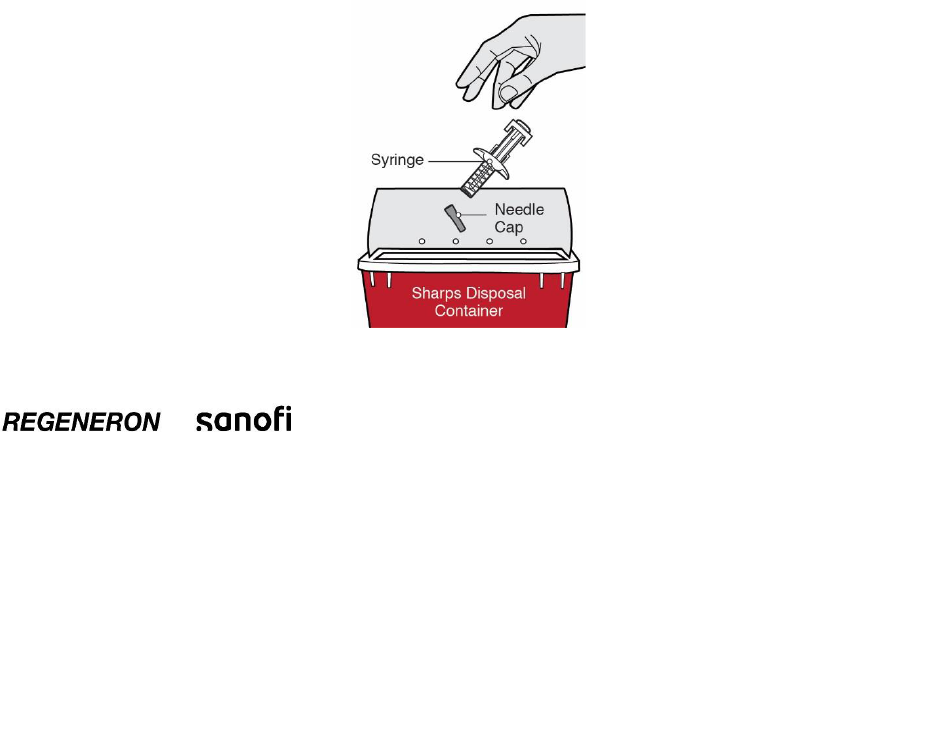
Step 13: Dispose
Put used Needles, DUPIXENT Syringes, and Needle Caps in a FDA-cleared sharps disposal
container right away after use.
Do not dispose of (throw away) Needles, DUPIXENT Syringes, and Needle Caps in
your household trash.
If you do not have a FDA-cleared sharps disposal container, you may use a household
container that is:
• made of a heavy-duty plastic,
• can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to
come out,
• upright and stable during use,
• leak-resistant, and
• properly labeled to warn of hazardous waste inside the container
When your sharps disposal container is almost full, you will need to follow your community
guidelines for the right way to dispose of your sharps disposal container. There may be state or
local laws about how you should throw away used Needles and Syringes.
For more information about safe sharps disposal, and for specific information about sharps
disposal in the state that you live in, go to the FDA’s website at:
http://www.fda.gov/safesharpsdisposal
Do not dispose of your used sharps disposal container in your household trash unless your
community guidelines permit this. Do not recycle your used sharps disposal container.
Do not put the Needle Cap back on.
Reference ID: 5208898
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Manufactured by: Regeneron Pharmaceuticals, Inc. Tarrytown, NY 10591
U.S. License No. 1760
Marketed by: sanofi-aventis U.S. LLC (Bridgewater, NJ 08807) and Regeneron Pharmaceuticals,
Inc. (Tarrytown, NY 10591)
DUPIXENT
®
is a registered trademark of Sanofi Biotechnology
© 2023 Regeneron Pharmaceuticals, Inc. / sanofi-aventis U.S. LLC. All rights reserved.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: July 2023
Reference ID: 5208898
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

INSTRUCTIONS FOR USE
DUPIXENT
®
(DU-pix-ent)
(dupilumab)
injection, for subcutaneous use
Single-Dose Pre-filled Syringe with Needle Shield
Read this Instructions for Use before using the DUPIXENT Pre-filled Syringe. Do not inject
yourself or someone else until you have been shown how to inject DUPIXENT. Your
healthcare provider can show you or your caregiver how to prepare and inject a dose of
DUPIXENT before you try to do it yourself the first time. Keep these instructions for future use.
Call your healthcare provider if you have any questions.
This device is a Single-Dose Pre-filled Syringe (called “DUPIXENT Syringe” in these
instructions). It contains 200 mg of DUPIXENT for injection under the skin (subcutaneous
injection).
The parts of the DUPIXENT Syringe are shown below:
Important Information
• Read all of the instructions carefully
before using the DUPIXENT Syringe.
• Ask your healthcare provider how often
you will need to inject the medicine.
• In children 12 years of age and older, it
is recommended that DUPIXENT be
administered by or under supervision of
an adult.
• In children 6 months to less than 12
years of age, DUPIXENT should be given
by a caregiver.
• Rotate the injection site each time you
inject.
• Do not use the DUPIXENT Syringe if it
has been dropped on a hard surface or
damaged.
• To reduce the risk of accidental needle
sticks, each pre-filled syringe has a
Needle Shield that is automatically
activated to cover the needle after you
have given your injection.
• Do not pull back on the Plunger Rod at
any time.
• Do not remove the Needle Cap until just
before you give the injection.
• Throw away (dispose of) the used
DUPIXENT Single-Dose Pre-filled
Syringe right away after use. See “Step
13: Dispose” below.
• Do not re-use a DUPIXENT Single-
Dose Pre-filled Syringe.
Reference ID: 5208898
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

• Do not use the DUPIXENT Syringe if the
Needle Cap is missing or not securely
attached.
• Do not touch the Plunger Rod until you
are ready to inject.
• Do not inject through clothes.
• Do not get rid of any air bubble in the
DUPIXENT Syringe.
How should I store DUPIXENT?
• Keep DUPIXENT Syringes and all medicines out of the reach of children.
• Store DUPIXENT Syringes in the refrigerator between 36°F to 46°F (2°C to 8°C).
• Store DUPIXENT Syringes in the original carton to protect them from light.
• DUPIXENT Syringes can be stored at room temperature up to 77°F (25°C) up to 14 days.
Throw away (dispose of) any DUPIXENT Syringes that have been left at room
temperature for longer than 14 days.
• Do not shake the DUPIXENT Syringe.
• Do not heat the DUPIXENT Syringe.
• Do not freeze the DUPIXENT Syringe.
• Do not put the DUPIXENT Syringe into direct sunlight.
Step 1: Remove
Remove the DUPIXENT Syringe from the carton by holding the middle of the Syringe Body.
Do not pull off the Needle Cap until you are ready to inject.
Do not use the DUPIXENT Syringe if it has been dropped on a hard surface or
damaged.
Step 2: Prepare
Ensure you have the following:
• the DUPIXENT Pre-filled Syringe
• 1 alcohol wipe*
• 1 cotton ball or gauze*
Reference ID: 5208898
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

• a sharps disposal container* (See Step 13)
*Items not included in the carton
Step 3: Check
When you receive your DUPIXENT Syringes, always check to see that:
• you have the correct medicine and dose.
• the expiration date on the Single-Dose Pre-filled Syringe has not passed.
Do not use the DUPIXENT Syringe if the expiration date has passed.
Step 4: Inspect
Look at the medicine through the Viewing Window on the DUPIXENT Syringe:
Check to see if the liquid is clear and colorless to pale yellow.
Note: You may see an air bubble, this is normal.
Do not use the DUPIXENT Syringe if the liquid is discolored or cloudy, or if it contains
visible flakes or particles.
Reference ID: 5208898
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Step 5: Wait 30 minutes
Lay the DUPIXENT Syringe on a flat surface and let it naturally warm to room temperature for at
least 30 minutes.
Do not heat the DUPIXENT Syringe.
Do not put the DUPIXENT Syringe into direct sunlight.
Do not keep DUPIXENT Syringes at room temperature for more than 14 days. Throw
away (dispose of) any DUPIXENT Syringes that have been left at room temperature
for longer than 14 days.
Step 6: Choose your injection site
• You can inject into your thigh or stomach, except for the 2 inches (5 cm) around your
belly button (navel).
• If a caregiver injects your dose, they can also use the outer area of the upper arm.
• Choose a different site each time you inject DUPIXENT.
Do not inject into skin that is tender, damaged, bruised or scarred.
Reference ID: 5208898
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Step 7: Clean
Wash your hands.
Clean the injection site with an alcohol wipe.
Let your skin dry before injecting.
Do not touch the injection site again or blow on it before the injection.
Step 8: Remove Needle Cap
Hold the DUPIXENT Syringe in the middle of the Syringe Body with the Needle pointing away
from you and pull off the Needle Cap.
Do not put the Needle Cap back on.
Reference ID: 5208898
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Do not touch the Needle.
Inject your medicine right away after removing the Needle Cap.
Step 9: Pinch
Pinch a fold of skin at the injection site (thigh or stomach, except 2 inches around your belly
button, or outer area of the upper arm if injected by your caregiver). The figure below shows an
example of pinching a fold of skin on your stomach.
Step 10: Insert
Insert the Needle completely into the fold of the skin at about a 45º angle.
Reference ID: 5208898
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Step 11: Push
Relax the pinch.
Push the Plunger Rod down slowly and steadily as far as it will go until the DUPIXENT Syringe
is empty.
Note: You will feel some resistance. This is normal.
Step 12: Release and Remove
Lift your thumb to release the Plunger Rod until the Needle is covered by the Needle Shield and
then remove the Syringe from the injection site.
Lightly press a cotton ball or gauze on the injection site if you see any blood.
Do not put the Needle Cap back on.
Do not rub your skin after the injection.
Reference ID: 5208898
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Step 13: Dispose
Put your used Needles, DUPIXENT Syringes, and Needle Caps in a FDA-cleared sharps
disposal container right away after use.
Do not dispose of (throw away) Needles, DUPIXENT Syringes, and Needle Caps in
your household trash.
If you do not have a FDA-cleared sharps disposal container, you may use a household
container that is:
• made of a heavy-duty plastic,
• can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to
come out,
• upright and stable during use,
• leak-resistant, and
• properly labeled to warn of hazardous waste inside the container
When your sharps disposal container is almost full, you will need to follow your community
guidelines for the right way to dispose of your sharps disposal container. There may be state or
local laws about how you should throw away used Needles and Syringes.
For more information about safe sharps disposal, and for specific information about sharps
disposal in the state that you live in, go to the FDA’s website at:
http://www.fda.gov/safesharpsdisposal
Do not dispose of your used sharps disposal container in your household trash unless your
community guidelines permit this. Do not recycle your used sharps disposal container.
Do not put the Needle Cap back on.
Reference ID: 5208898
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
Manufactured by: Regeneron Pharmaceuticals, Inc. Tarrytown, NY 10591
U.S. License No. 1760
Marketed by: sanofi-aventis U.S. LLC (Bridgewater, NJ 08807) and Regeneron Pharmaceuticals,
Inc. (Tarrytown, NY 10591)
DUPIXENT
®
is a registered trademark of Sanofi Biotechnology
© 2023 Regeneron Pharmaceuticals, Inc. / sanofi-aventis U.S. LLC. All rights reserved.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: July 2023
Reference ID: 5208898
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Instructions for Use
DUPIXENT
®
(DU-pix-ent)
(dupilumab)
injection, for subcutaneous use
Single-Dose Pre-filled Pen (200 mg/1.14 mL)
This Instructions for Use contains information on how to inject DUPIXENT.
Read this Instructions for Use before using the DUPIXENT Pre-filled Pen. Do not inject yourself or someone else until
you have been shown how to inject DUPIXENT. Your healthcare provider can show you or your caregiver how to
prepare and inject a dose of DUPIXENT before you try to do it yourself for the first time. Keep this Instructions for Use.
Call your healthcare provider if you have any questions.
This DUPIXENT Pre-filled Pen is only for use in adults and children aged 2 years and older.
This DUPIXENT Pre-filled Pen is a single-dose device. It contains 200 mg of DUPIXENT for injection under the skin
(subcutaneous injection).
The parts of the DUPIXENT Pre-filled Pen are shown below:
Important Information
• Read all of the instructions carefully before using the DUPIXENT Pre-filled Pen.
• Ask your healthcare provider how often you will need to inject the medicine.
• In children 12 years of age and older, it is recommended that DUPIXENT be administered by or under
supervision of an adult.
• In children 2 years to less than 12 years of age, DUPIXENT should be given by a caregiver.
• Choose a different injection site for each injection.
• Do not press or touch the Orange Needle Cover with your fingers.
• Do not inject through clothes.
• Do not remove the Yellow Cap until just before you give the injection.
• Do not try to put the Yellow Cap back on the DUPIXENT Pre-filled Pen.
• Throw away (dispose of) the used DUPIXENT Pre-filled Pen right away after use.
• Do not re-use a DUPIXENT Pre-filled Pen.
Reference ID: 5208898
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Storing DUPIXENT
• Store unused DUPIXENT Pre-filled Pens in the refrigerator between 36ºF to 46ºF (2ºC to 8ºC).
• Store DUPIXENT Pre-filled Pens in the original carton to protect from light.
• If necessary, you may keep DUPIXENT Pre-filled Pens at room temperature up to 77ºF (25ºC) for up to 14
days.
• Do not store DUPIXENT Pre-filled Pens at room temperature more than 77ºF (25ºC).
• After removing a DUPIXENT Pre-filled Pen from the refrigerator, it must be used within 14 days or thrown
away (disposed of).
• Do not shake the DUPIXENT Pre-filled Pen at any time.
• Do not heat the DUPIXENT Pre-filled Pen.
• Do not freeze the DUPIXENT Pre-filled Pen.
• Do not put the DUPIXENT Pre-filled Pen into direct sunlight.
• Keep DUPIXENT Pre-filled Pens and all medicines out of the reach of children.
A. Get ready to inject
A1. Gather supplies
Find a clean, flat work surface.
Make sure you have the following
supplies:
A2. Check the Pen
• Do not use the DUPIXENT
Pre-filled Pen if it has been
damaged.
• Do not use the DUPIXENT
Pre-filled Pen if the Yellow Cap
is missing or not securely
attached.
• Do not use the DUPIXENT
Pre-filled Pen if the Window is
yellow before use.
A3. Look at the Label
• Check to be sure that you have
the correct Medicine and dose.
Reference ID: 5208898
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

• Check the expiration date.
• Do not use the DUPIXENT
Pre-filled Pen if the expiration
date has passed.
A4. Check the Medicine
• Look at the Medicine through
the Window: it should be clear
and colorless to pale yellow.
• Note: You may see an air
bubble, this is normal.
• Do not use the
DUPIXENT Pre-filled Pen if the
liquid is discolored or cloudy,
or if it contains visible flakes or
particles.
A5. Wait 30 minutes
• Lay the DUPIXENT Pre-filled
Pen on a flat surface and let it
warm up at room temperature
less than 77ºF (25ºC) for at
least 30 minutes.
• Do not heat the
DUPIXENT Pre-filled Pen in a
microwave, hot water or direct
sunlight.
Reference ID: 5208898
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

B. Choose and prepare your injection site
B1. Wash your hands well with soap and water
B2. Choose an injection site
• Thigh
• Stomach except for the 2
inches (5 cm) around your
belly button (navel).
• A caregiver can also inject in
the outer area of the upper
arm.
• Choose a different site for
each injection.
• Do not inject into skin
that is tender, damaged, has
bruises or scars, or into areas
with visible veins.
• Do not inject through
clothes.
Reference ID: 5208898
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
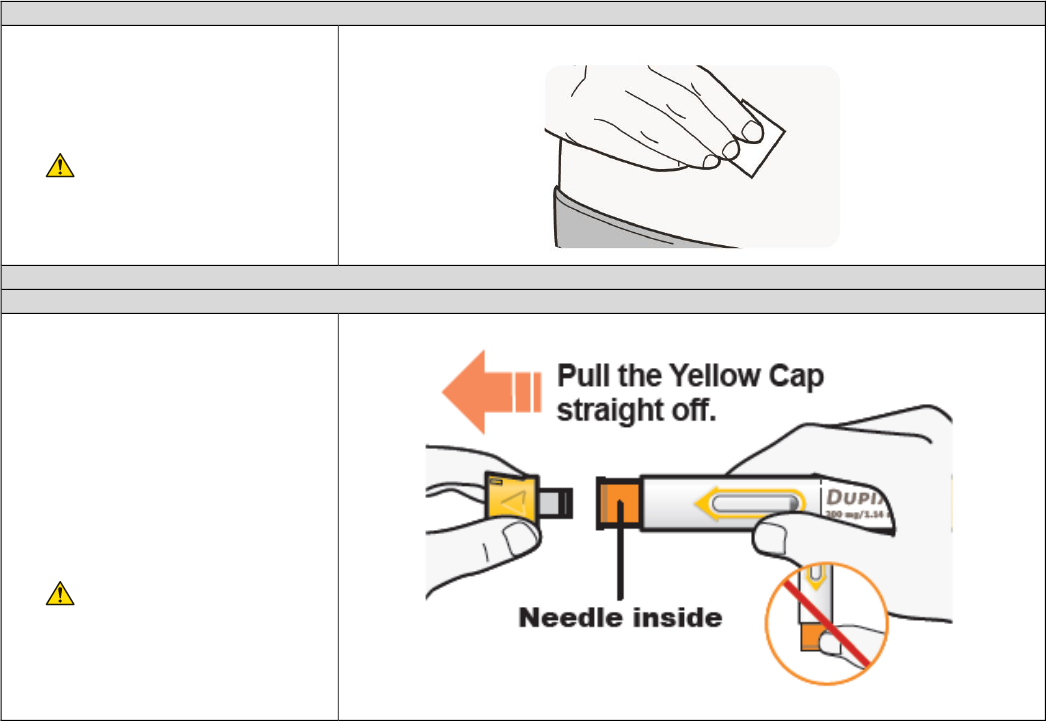
B3. Prepare the injection site
• Clean the injection site with an
alcohol wipe.
• Let the skin dry before injecting.
• Do not touch the injection
site again or blow on it before
the injection.
C. Give the injection
C1. Remove Yellow Cap
• Remove the Yellow Cap by
pulling it straight off, as shown.
Do not twist the Yellow Cap off.
• Do not remove the Yellow Cap
until you are ready to inject.
• Do not press or touch the
Orange Needle Cover with your
fingers. The Needle is inside.
• Do not put the Yellow Cap
back on the DUPIXENT Pre-
filled Pen after you have
removed it.
Reference ID: 5208898
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

C2. Pinch Skin and Place
Pinch the skin before and during the injection.
• Hold the DUPIXENT Pre-filled Pen as shown so that you can see the Window. Place the Orange Needle Cover
on the skin.
• Place the Orange Needle Cover on the skin at approximately a 90-degree angle.
• Pinching is not needed for adults and children aged 12 years and older.
Reference ID: 5208898
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

C3. Press down → Watch Window turn fully yellow → Then count to 5
Press and hold the DUPIXENT Pre-
filled Pen firmly against the skin
until you cannot see the Orange
Needle Cover.
• There will be a “click” when the
injection starts, and
• The Window will start to turn
yellow.
• Keep pressing the DUPIXENT
Pre-filled Pen against the skin.
Keep pressing the DUPIXENT Pre-
filled Pen against the skin and watch
the window:
• The Window will turn completely
yellow, and
• You will hear a 2nd “click”.
• Keep pressing the DUPIXENT
Pre-filled Pen against the skin.
Keep pressing the DUPIXENT Pre-
filled Pen against the skin and count
to 5 to make sure you get your full
dose.
Pinching of the skin is not needed for adults and children aged 12 years and older.
Reference ID: 5208898
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

C4. Remove
• After you have completed the
injection pull straight up to
remove DUPIXENT Pre-filled
Pen from the skin. The Orange
Needle Cover will cover the
needle.
• If you see any blood at the site,
lightly dab a cotton ball or gauze
pad.
• Do not rub the skin after
the injection.
• If the Window does not turn
completely Yellow, or if it looks
like medicine is still coming out
of the pen, you may not have
received a full dose. Dispose of
(throw away) the pen and
contact your healthcare provider
right away.
• Do not give a second dose
without speaking to your
healthcare provider.
Reference ID: 5208898
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
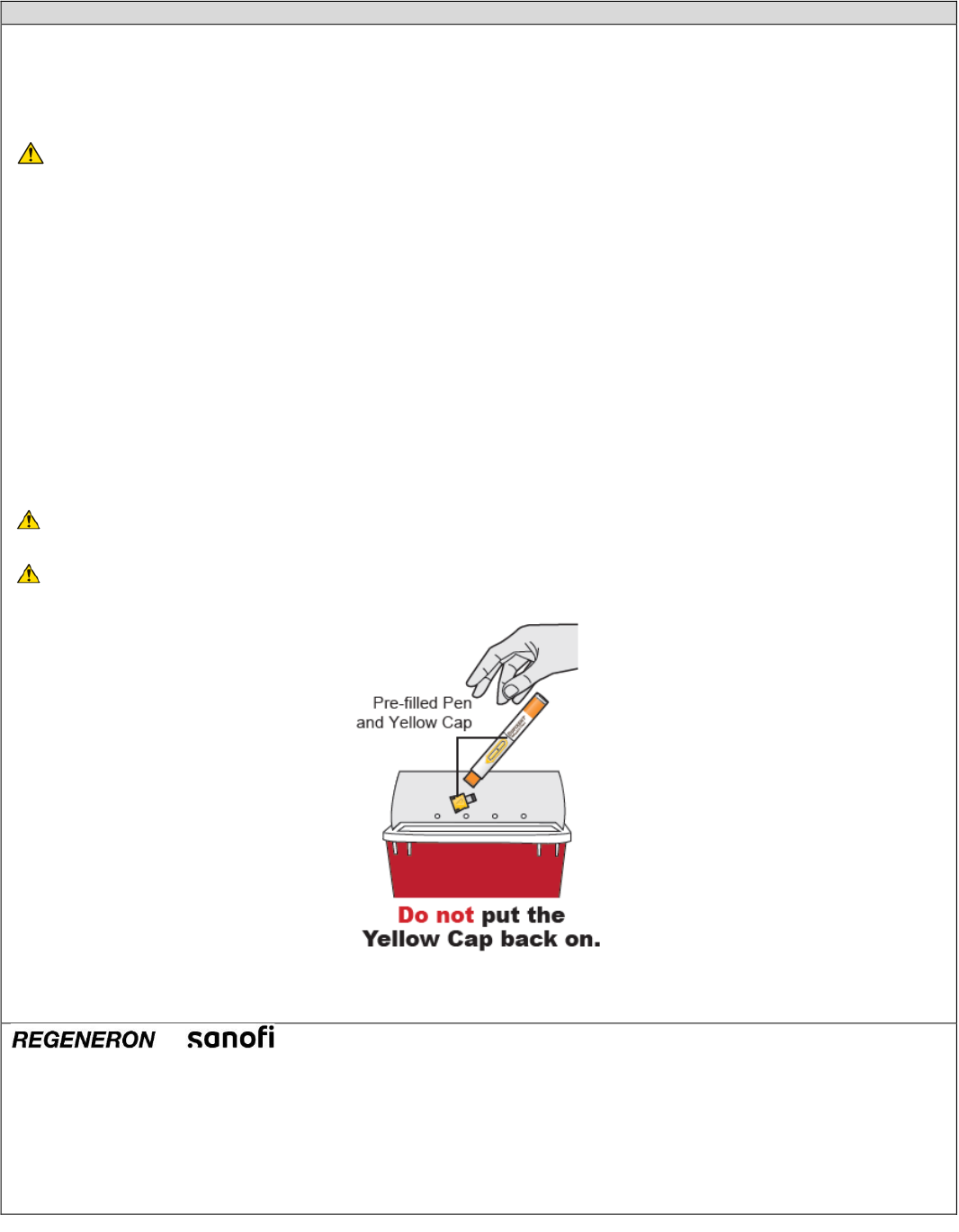
D. Dispose of used DUPIXENT Pre-filled Pen
How to Dispose of (throw away) DUPIXENT Pre-filled Pen:
Put the used DUPIXENT Pre-filled Pens and Yellow Caps in a FDA-cleared sharps disposal container right away
after use.
Do not dispose of (throw away) DUPIXENT Pre-filled Pens and Yellow Caps in your household trash.
If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
• made of a heavy-duty plastic,
• can be closed with a tightfitting, puncture-resistant lid, without sharps being able to come out,
• upright and stable during use,
• leak-resistant, and
• properly labeled to warn of hazardous waste inside the container.
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right
way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away
used DUPIXENT Pre-filled Pens.
For more information about safe sharps disposal, and for specific information about sharps disposal in the state that
you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal
Do not dispose of your used sharps disposal container in your household trash unless your community
guidelines permit this.
Do not recycle your used sharps disposal container.
Keep your sharps disposal container out of the reach of children.
Manufactured by:
Regeneron Pharmaceuticals, Inc.
Tarrytown, NY 10591
U.S. License No. 1760
Marketed by:
sanofi-aventis U.S. LLC (Bridgewater, NJ 08807) and
Regeneron Pharmaceuticals, Inc. (Tarrytown, NY 10591)
DUPIXENT
®
is a registered trademark of Sanofi Biotechnology
© 2023 Regeneron Pharmaceuticals, Inc. / sanofi-aventis U.S. LLC. All rights reserved.
This Instructions for Use has been approved by the U.S. Food and Drug Administration. Revised: July 2023
Reference ID: 5208898
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

INSTRUCTIONS FOR USE
DUPIXENT
®
(DU-pix-ent)
(dupilumab)
injection, for subcutaneous use
Single-Dose Pre-filled Syringe with Needle Shield
Read this Instructions for Use before using the DUPIXENT Pre-filled Syringe. Do not inject
yourself or someone else until you have been shown how to inject DUPIXENT. Your
healthcare provider can show you or your caregiver how to prepare and inject a dose of
DUPIXENT before you try to do it yourself the first time. Keep these instructions for future use.
Call your healthcare provider if you have any questions.
This device is a Single-Dose Pre-filled Syringe (called “DUPIXENT Syringe” in these
instructions). It contains 300 mg of DUPIXENT for injection under the skin (subcutaneous
injection).
The parts of the DUPIXENT Syringe are shown below:
Important Information
Read all of the instructions carefully
before using the DUPIXENT Syringe.
Ask your healthcare provider how often
you will need to inject the medicine.
In children 12 years of age and older,
it
is recommended that DUPIXENT be
administered by or under supervision of
an adult.
In children 6 months to less than 12
years of age, DUPIXENT should be
given by a caregiver.
Rotate the injection site each time you
inject.
To reduce the risk of accidental needle
sticks, each pre-filled syringe has a
Needle Shield that is automatically
activated to cover the needle after you
have given your injection.
Do not pull back on the Plunger Rod at
any time.
Do not remove the Needle Cap until just
before you give the injection.
Throw away (dispose of) the used
DUPIXENT Single-Dose Pre-filled
Syringe right away after use. See “Step
13: Dispose” below.
Reference ID: 5208898
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Do not use the DUPIXENT Syringe if it
has been dropped on a hard surface or
damaged.
Do not use the DUPIXENT Syringe if the
Needle Cap is missing or not securely
attached.
Do not touch the Plunger Rod until you
are ready to inject.
Do not inject through clothes.
Do not get rid of any air bubble in th
e
DUPIXENT Syringe.
Do not re-use a DUPIXENT Single-
Dose Pre-filled Syringe.
How should I store DUPIXENT?
Keep DUPIXENT Syringes and all medicines out of the reach of children.
Store DUPIXENT Syringes in the refrigerator between 36°F to 46°F (2°C to 8°C).
Store DUPIXENT Syringes in the original carton to protect them from light.
DUPIXENT Syringes can be stored at room temperature up to 77°F (25°C) up to 14 days.
Throw away (dispose of) any DUPIXENT Syringes that have been left at room
temperature for longer than 14 days.
Do not shake the DUPIXENT Syringe.
Do not heat the DUPIXENT Syringe.
Do not freeze the DUPIXENT Syringe.
Do not put the DUPIXENT Syringe into direct sunlight.
Step 1: Remove
Remove the DUPIXENT
Syringe from
the carton by holding the middle of the Syringe Body.
Do not pull off the Needle Cap until you are ready to inject.
Do not use the DUPIXENT Syringe if it has been dropped on a hard surface or
damaged.
Step 2: Prepare
Ensure you have the following:
Reference ID: 5208898
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

the DUPIXENT Pre-filled Syringe
1 alcohol wipe*
1 cotton ball or gauze*
a sharps disposal container* (See Step 13)
*Items not included in the carton
Step 3: Check
When you receive your DUPIXENT Syringes, always check to see that:
you have the correct medicine and do
se.
the expiration date on the Single-Dose Pre-filled Syringe has not passed.
Do not use the DUPIXENT
Syringe if the expiration date has passed.
Step 4: Inspect
Look at the medicine through the Viewing Window on the DUPIXENT Syringe:
Check to see if the liquid is clear and colorless to pale yellow.
Note: You may see an air bubble, this is normal.
Do not use the DUPIXENT Syringe if the liquid is discolored or cloudy, or if it contains
visible flakes or particles.
Reference ID: 5208898
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Step 5: Wait 45 minutes
Lay the DUPIXENT Syringe on a fla
t surface and let it natur
ally warm to room temperature for at
least 45 minutes.
Do not heat the DUPIXENT Syringe.
Do not put the DUPIXENT Syringe into direct sunlight.
Do not keep DUPIXENT Syringes at room temperature for more than 14 days. Throw
away (dispose of) any DUPIXENT Syringes that have been left at room temperature
for longer than 14 days.
Step 6: Choose your injection site
You can inject into your thigh or stomach, except
for the 2 inches (5 cm) around your
belly button (navel).
If a caregiver injects your dose, they can also use the outer area of the
upper arm.
Choose a different site each time you inject DUPIXENT.
Do not inject into skin that is tender, damaged, bruised or scarred.
Reference ID: 5208898
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Step 7: Clean
Wash your
hands.
Clean the inj
ection site with an alcohol wipe.
Let your skin dry before injecting.
Do not touch the injection site again or blow on it before the injection.
Step 8: Remove Needle Cap
Hold the DUPIXENT Syringe in the middle of the Syringe Body with the Needle pointing away
from you and pull off the Needle Cap.
Do not put the Needle Cap back on.
Reference ID: 5208898
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Do not touch the Needle.
Inject your medicine right away after removing the Needle Cap.
Step 9: Pinch
Pinch a fold of skin at the injection site (thigh or stomach, except 2 inches around your belly
button, or outer area of the upper arm if injected by your caregiver). The figure below shows an
example of pinching a fold of skin on your stomach.
Step 10: Insert
Insert the Needle completely into the fold of the skin at about a 45º angle.
Reference ID: 5208898
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Step 11: Push
Relax the pinch.
Push the Plunger Rod down slowly
and steadily
as far as it will go until the DUPIXENT Syringe
is empty.
Note: You will feel some resistance. This is normal.
Step 12: Release and Remove
Lift your thumb to release the Plunger Rod until the Needle is covered by the Needle Shield and
then remove the Syringe from the injection site.
Lightly press a cotton ball or gauze on the injection site if you see any blood.
Do not put the Needle Cap back on.
Reference ID: 5208898
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Do not rub your skin after the injection.
Step 13: Dispose
Put your used Needles, DUPIXENT Syringes, and Needle Caps in a FDA-cleared sharps
disposal container right away after use.
Do not dispose of (throw away) Needles, DUPIXENT Syringes, and Needle Caps in
your household trash.
If you do not have a FDA-cleared sharps disposal container, you may use a household
container that is:
made of a heavy-duty plastic,
can be closed with a tight-fitting, puncture-resistant lid, without sharps be
ing able to
come out,
upright and stable during use,
leak-resistant, and
properly labeled to warn of hazardous waste inside the container
When your sharps disposal container is almost full, you will need to follow your community
guidelines for the right way to dispose of your sharps disposal container. There may be state or
local laws about how you should throw away used Needles and Syringes.
For more information about safe sharps disposal, and for specific information about sharps
disposal in the state that you live in, go to the FDA’s website at:
http://www.fda.gov/safesharpsdisposal
Do not dispose of your used sharps disposal container in yo
ur household trash unless your
community guidelines permit this. Do not recycle your used sharps disposal container.
Do not put the Needle Cap back on.
Reference ID: 5208898
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Manufactured by: Regeneron Pharmaceuticals, Inc. Tarrytown, NY 10591
U.S. License No. 1760
Marketed by: sanofi-aventis U.S. LLC (Bridgewa
ter, NJ 08807) and Regeneron Pharmaceuticals,
Inc. (Tarrytown, NY 10591)
DUPIXENT
®
is a registered trademark of Sanofi Biotechnology
© 2023 Regeneron Pharmaceuticals, Inc. / sanofi-aventis U.S. LLC. All rights reserved.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: July 2023
Reference ID: 5208898
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Instructions for Use
DUPIXENT
®
(DU-pix-ent)
(dupilumab)
injection, for subcutaneous use
Single-Dose Pre-filled Pen (300 mg/2 mL)
This Instructions for Use contains information on how to inject DUPIXENT.
Read this Instructions for Use before using the DUPIXENT Pre-filled Pen. Do not inject yourself or someone else until
you have been shown how to inject DUPIXENT. Your healthcare provider can show you or your caregiver how to
prepare and inject a dose of DUPIXENT before you try to do it yourself for the first time. Keep this Instructions for Use.
Call your healthcare provider if you have any questions.
This DUPIXENT Pre-filled Pen is only for use in adults and children aged 2 years and older.
This DUPIXENT Pre-filled Pen is a single-dose device. It contains 300 mg of DUPIXENT for injection under the skin
(subcutaneous injection).
The parts of the DUPIXENT Pre-filled Pen are shown below:
Important Information
• Read all of the instructions carefully before using the DUPIXENT Pre-filled Pen.
• Ask your healthcare provider how often you will need to inject the medicine.
• In children 12 years of age and older, it is recommended that DUPIXENT be administered by or under
supervision of an adult.
• In children 2 years to less than 12 years of age, DUPIXENT should be given by a caregiver.
• Choose a different injection site for each injection.
• Do not press or touch the Yellow Needle Cover with your fingers.
• Do not inject through clothes.
• Do not remove the Green Cap until just before you give the injection.
• Do not try to put the Green Cap back on the DUPIXENT Pre-filled Pen.
• Throw away (dispose of) the used DUPIXENT Pre-filled Pen right away after use.
• Do not re-use a DUPIXENT Pre-filled Pen.
Reference ID: 5208898
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Storing DUPIXENT
• Store unused DUPIXENT Pre-filled Pens in the refrigerator between 36ºF to 46ºF (2ºC to 8ºC).
• Store DUPIXENT Pre-filled Pens in the original carton to protect from light.
• If necessary, you may keep DUPIXENT Pre-filled Pens at room temperature up to 77ºF (25ºC) for up to 14
days.
• Do not store DUPIXENT Pre-filled Pens at room temperature more than 77ºF (25ºC).
• After removing a DUPIXENT Pre-filled Pen from the refrigerator, it must be used within 14 days or thrown away
(disposed of).
• Do not shake the DUPIXENT Pre-filled Pen at any time.
• Do not heat the DUPIXENT Pre-filled Pen.
• Do not freeze the DUPIXENT Pre-filled Pen.
• Do not put the DUPIXENT Pre-filled Pen into direct sunlight.
• Keep DUPIXENT Pre-filled Pens and all medicines out of the reach of children.
A. Get ready to inject
A1. Gather supplies
Find a clean, flat work surface. Make
sure you have the following supplies:
A2. Check the Pen
• Do not use the DUPIXENT Pre-
filled Pen if it has been damaged.
• Do not use the DUPIXENT Pre-
filled Pen if the Green Cap is
missing or not securely attached.
• Do not use the DUPIXENT Pre-
filled Pen if the Window is yellow
before use.
A3. Look at the Label
• Check to be sure that you have the
correct Medicine and dose.
Reference ID: 5208898
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

• Check the expiration date.
• Do not use the DUPIXENT
Pre-filled Pen if the expiration date
has passed.
A4. Check the Medicine
• Look at the Medicine through the
Window: it should be clear and
colorless to pale yellow.
• Note: You may see an air bubble,
this is normal.
• Do not use the DUPIXENT
Pre-filled Pen if the liquid is
discolored or cloudy, or if it contains
visible flakes or particles.
A5. Wait 45 minutes
• Lay the DUPIXENT Pre-filled Pen
on a flat surface and let it warm up
at room temperature less than 77ºF
(25ºC) for at least 45 minutes.
• Do not heat the DUPIXENT
Pre-filled Pen in a microwave, hot
water or direct sunlight.
Reference ID: 5208898
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

B. Choose and prepare your injection site
B1. Wash your hands well with soap and water
B2. Choose an injection site
• Thigh
• Stomach except for the 2 inches (5
cm) around your belly button
(navel).
• A caregiver can also inject in the
outer area of the upper arm.
• Choose a different site for each
injection.
• Do not inject into skin that is
tender, damaged, has bruises or
scars, or into areas with visible
veins.
• Do not inject through clothes.
Reference ID: 5208898
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

B3. Prepare the injection site
• Clean the injection site with an
alcohol wipe.
• Let the skin dry before injecting.
• Do not touch the injection site
again or blow on it before the
injection.
C. Give the injection
C1. Remove Green Cap
• Remove the Green Cap by pulling it
straight off, as shown. Do not twist
the Green Cap off.
• Do not remove the Green Cap until
you are ready to inject.
• Do not press or touch the Yellow
Needle Cover with your fingers. The
Needle is inside.
• Do not put the Green Cap
back on the DUPIXENT Pre-filled
Pen after you have removed it.
Reference ID: 5208898
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

C2. Pinch Skin and Place
Pinch the skin before and during the injection.
• Hold the DUPIXENT Pre-filled Pen as shown so that you can see the Window. Place the Yellow Needle Cover on the
skin.
• Place the Yellow Needle Cover on the skin at approximately a 90-degree angle.
• Pinching is not needed for adults and children aged 12 years and older.
Reference ID: 5208898
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

C3. Press down → Watch Window turn fully yellow → Then count to 5
Press and hold the DUPIXENT Pre-
filled Pen firmly against the skin
until you cannot see the Yellow
Needle Cover.
• There will be a “click” when the
injection starts, and
• The Window will start to turn
yellow.
• Keep pressing the DUPIXENT
Pre-filled Pen against the skin.
Keep pressing the DUPIXENT Pre-
filled Pen against the skin and watch
the window:
• The Window will turn completely
yellow, and
• You will hear a 2nd “click”.
• Keep pressing the DUPIXENT Pre-
filled Pen against the skin.
Keep pressing the DUPIXENT Pre-
filled Pen against the skin and count to
5 to make sure you get your full
dose.
Pinching of the skin is not needed for adults and children aged 12 years and older.
Reference ID: 5208898
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
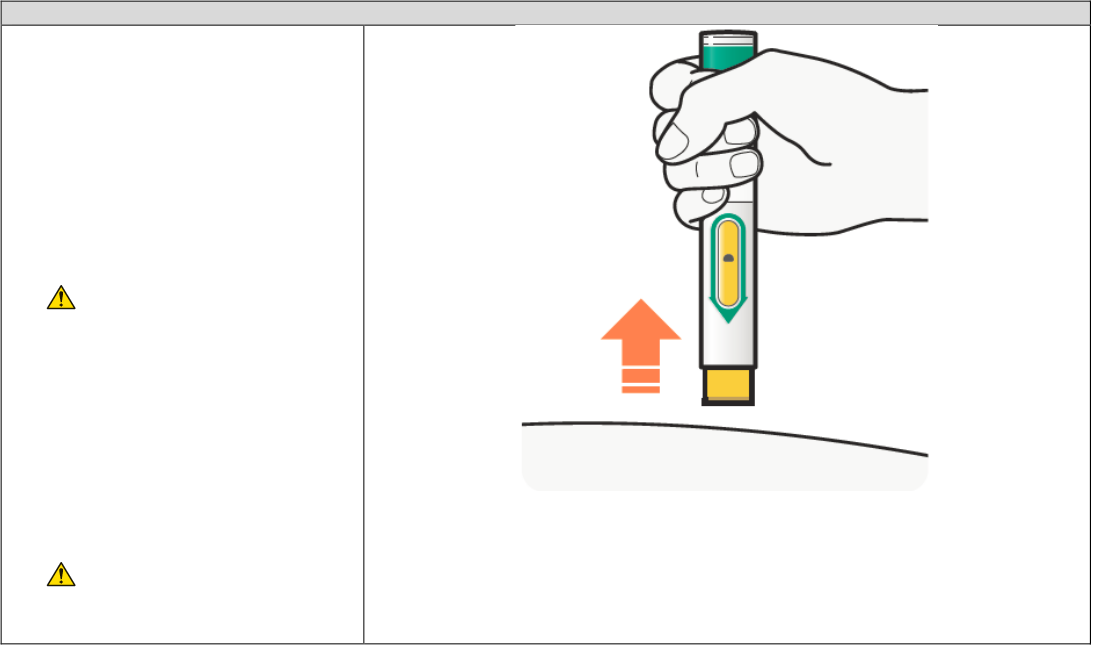
C4. Remove
• After you have completed the
injection pull straight up to remove
DUPIXENT Pre-filled Pen from the
skin. The Yellow Needle Cover will
cover the needle.
• If you see any blood at the site,
lightly dab a cotton ball or gauze
pad.
• Do not rub the skin after the
injection.
• If the Window does not turn
completely Yellow, or if it looks like
medicine is still coming out of the
pen, you may not have received a
full dose. Dispose of (throw away)
the pen and contact your
healthcare provider right away.
• Do not give a second dose
without speaking to your
healthcare provider.
Reference ID: 5208898
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

D. Dispose of used DUPIXENT Pre-filled Pen
How to Dispose of (throw away) DUPIXENT Pre-filled Pen:
Put the used DUPIXENT Pre-filled Pens and Green Caps in a FDA-cleared sharps disposal container right away after
use.
Do not dispose of (throw away) DUPIXENT Pre-filled Pens and Green Caps in your household trash.
If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
• made of a heavy-duty plastic,
• can be closed with a tightfitting, puncture-resistant lid, without sharps being able to come out,
• upright and stable during use,
• leak-resistant, and
• properly labeled to warn of hazardous waste inside the container.
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to
dispose of your sharps disposal container. There may be state or local laws about how you should throw away used
DUPIXENT Pre-filled Pens.
For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you
live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal
Do not dispose of your used sharps disposal container in your household trash unless your community guidelines
permit this.
Do not recycle your used sharps disposal container.
Keep your sharps disposal container out of the reach of children.
Manufactured by:
Regeneron Pharmaceuticals, Inc.
Tarrytown, NY 10591
U.S. License No. 1760
Marketed by:
sanofi-aventis U.S. LLC (Bridgewater, NJ 08807) and
Regeneron Pharmaceuticals, Inc. (Tarrytown, NY 10591)
DUPIXENT
®
is a registered trademark of Sanofi Biotechnology
© 2023 Regeneron Pharmaceuticals, Inc. / sanofi-aventis U.S. LLC. All rights reserved.
This Instructions for Use has been approved by the U.S. Food and Drug Administration. Revised: July 2023
Reference ID: 5208898
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
