
429
Robert Ménard (ed.), Malaria: Methods and Protocols, Methods in Molecular Biology, vol. 923,
DOI 10.1007/978-1-62703-026-7_30, © Springer Science+Business Media, LLC 2013
Chapter 30
Quantitative Analysis of Plasmodium berghei Liver Stages
by Bioluminescence Imaging
Takeshi Annoura , Séverine Chevalley , Chris J. Janse ,
Blandine Franke-Fayard , and Shahid M. Khan
Abstract
We describe simple and sensitive in vitro and in vivo assays to analyze Plasmodium liver stage development
using transgenic P. berghei parasites ( Pb GFP-Luc
con
), which express the bioluminescent reporter protein,
luciferase. In these assays, parasite development in hepatocytes is visualized and quanti fi ed by real-time
bioluminescence imaging both in culture and in live mice. We also describe quanti fi cation of in vitro liver-
stage development by measuring luminescence using a microplate reader. Reporter-parasite based
quanti fi cation of liver-stage development is faster and correlates very well with established quantitative
RT-PCR methods currently used to assess parasite development inside hepatocytes, both in live mice and
in culture.
Key words: Malaria , Plasmodium berghei , Sporozoites , Preerythrocytic stages , Liver , Hepatocytes ,
Luciferase , Luminescence , In vivo imaging , Drug screening , Mice
Quantitative analysis of Plasmodium liver-stage development both
in vivo in laboratory rodents and in vitro in cultured liver cells is
hampered by the low levels of parasite infection and by the compli-
cated methods required to monitor parasite growth. Currently,
one of the standard ways to assess drug ef fi cacy against liver stages
is to monitor liver stage development, both in vitro and in vivo, by
quantitative RT-PCR (qRT-PCR) methods (
1– 5 ) , and this is both
time-consuming and expensive. Other studies have involved assess-
ing parasite viability and direct quanti fi cation of development by
microscopy (
6 ) , RNA hybridization ( 7 ) , or infrared fl uorescence
1. Introduction

430 T. Annoura et al.
scanning system ( 8 ) . However, these methods not only are prone
to large variations between observers but are also time-consuming
given the very low infection rates (generally less than 2%) observed
in cultured hepatocytes (
8 ) . Here we describe simple and sensitive
in vitro and in vivo assays to visualize and quantify liver-stage devel-
opment using the transgenic P. berghei parasites Pb GFP-Luc
con
,
which expresses the bioluminescent reporter protein, luciferase.
The luminescence-based quanti fi cation of parasite development in
hepatocytes has been shown to correlate very well with established
quantitative RT-PCR methods (
9 ) . Speci fi cally, analysis of liver
infections by whole-body real-time imaging correlates well with
quantitative RT-PCR analysis of extracted livers. In addition lumi-
nescence-based quanti fi cation of liver stage parasites in cultured
hepatocytes by real-time imaging or using a microplate reader also
correlates well with quantitative RT-PCR methods. Both the
in vitro and in vivo liver imaging assays are amenable to screen
inhibitors and vaccines against liver stages (
9, 10 ) . Real-time imag-
ing of liver stages in mice has been successfully used to examine
host factors regulating liver infections and to monitor liver-stage
development of genetically attenuated parasites (
11 ) . Importantly,
the in vivo imaging assays allow the course of an infection to be
monitored, both throughout liver-stage parasite development and
in the blood stage of infection without sacri fi cing the animal, and
therefore, can greatly reduce the number of experimental animals
required to determine drug sensitivity. The simplicity and speed of
quantitative analysis of liver-stage development by real-time imag-
ing compared to the PCR-based methodologies, as well as the pos-
sibility to analyze parasite development in live mice without surgery,
should greatly enhance and simplify analyzing the effect of drugs
and vaccines on the liver stage of Plasmodium .
For the assays the reporter parasite line Pb GFP-Luc
con
(676m1cl1)
is used, which expresses a fusion protein of GFP (mutant3) and
fi re fl y luciferase (LUC-IAV) under the control of the constitutive
eef1 a promoter (
12 ) . For details of Pb GFP-Luc
con
, see RMgm-29
(
http://www.p berghei .eu/index.php?rmgm=29 ).
In our laboratory, mice of the following two strains are routinely
used: Swiss (OF1 ico, Construct 242, aged 6 weeks, 25–26 g) and
C57BL/6 (C57Bl/6Jico OF1, Construct 1, aged 6 weeks,
20–25 g). The mice are obtained from Charles River. Other (strains
or transgenic) mice can also be used for in vivo imaging experi-
ments (see Note 1).
2. Materials
2.1. Reporter Parasite
2.2. Laboratory
Animals
43130 Quantitative Analysis of Plasmodium berghei Liver Stages…
1. Sporozoites of parasite line Pb GFP-Luc
con
. This protocol
requires the collection of (large numbers) of sporozoites.
Sporozoites are removed from the salivary glands of infected
Anopheles stephensi mosquitoes at days 20–28 after feeding on
mice infected with Pb GFP-Luc
con
parasites. For procedures of
maintenance/rearing of mosquitoes and infection of mosqui-
toes, we refer to ref.
(13) .
2. Hepatocytes. The human hepatocyte carcinoma cell line Huh7
(JCRB0403, JCRB Cell Bank, JP) is used for in vitro cultures
of the liver stages.
3. Fetal bovine serum, heat-inactivated (FBS; Invitrogen; cat. no.
10108-165). Store at −20°C.
4. Phosphate-buffered saline (PBS). PBS stock solution (10×):
0.01 M KH
2
PO
4
, 0.1 M Na
2
HPO
4
, 1.37 M NaCl, 0.027 M
KCl, pH 7.4. For a working solution, dilute the stock solution
with 9 volumes of distilled water, adjust the pH to 7.2 with
1.0 M HCl and sterilize by autoclaving for 20 min at 120°C.
5. Complete RPMI1640 culture medium. RPMI1640 medium
(Invitrogen; cat. no. 31870-025) supplemented with FBS to a
fi nal concentration of 10 or 20% (v/v), 1% GlutaMAX
(Invitrogen; cat. no. 35050) and 1% penicillin–streptomycin
(MP Bio; cat. no. 1670049).
6. Trypsin, 0.05% (1×) with EDTA 4Na (Invitrogen; cat. no.
25300-054).
7. Cell culture lysis reagent (CCLR): Luciferase Assay System kit
(Promega, cat. no. E1500). For working solution, dilute the
“Cell Culture Lysis 5× Reagent” provided in the kit with
Milli-Q water.
8. Luciferase assay substrate solution: Luciferase Assay System Kit
(Promega, cat. no. E1500). For working solution, mix 1 vial of
luciferase assay substrate and 1 vial of 10 ml luciferase assay
buffer together. The mixed solution can be stored at −20°C
and can be subsequently freeze/thawed multiple times with-
out a signi fi cant loss of activity, however, the solution must be
kept in the dark at all times.
9.
D -Luciferin sodium salt. Dissolve 1 g D -luciferin in 12.5 ml
PBS to give a stock solution of 80 mg/ml and store at −20°C
in the dark in 500- m l aliquots. Thaw the stock solution prior to
use and inject into a mouse at a concentration of 120 mg/kg
body weight (i.e., 30 m l for a mouse of 20 g).
10. Inhibitors/antimalarial drugs: dissolve the powders in DMSO,
sterile Milli-Q water or culture medium in high concentration
as stock solution. Store at 4°C or −20°C. For serial dilutions,
dilute the working stock solutions with DMSO and/or culture
medium (see Note 2).
2.3. Reagents
432 T. Annoura et al.
1. Stereomicroscope (Leica M80) for mosquito dissection. Most
stereomicroscopes are suitable.
2. (Upright) light microscope (Leica DM2500 or Carl Zeiss
Standard 25 Zeiss) for counting sporozoites. All light micro-
scopes with 40× objective are suitable.
3. Inverted microscope, Leica DMIL for analysis of Huh7 cell
cultures. All inverted microscopes with 20× objective are
suitable.
4. Carbon dioxide gas source (for anesthesia of mosquitoes).
5. Precision forceps (Original Swiss Dumont precision forceps,
cat. no. K342.1). Most thin precision forceps are suitable.
6. Insulin needle syringe (BD Micro-Fine + U-100 insulin,
0.33 mm; 30 G × 8 mm, BD Medical, France). Most thin small
needle syringes are suitable.
7. Incubation (moist) chamber (COSMO BIO CO., LTD. cat.
no. KMB-10CG). All moist chambers are suitable.
8. Bürker-Türk counting chamber (Carl Roth GmbH, cat. no.
T730.1).
9. Tissue grinder of Polypropylene Pestle for 1.5-ml tubes (Carl
Roth GmbH, cat. no. P987.1).
10. 75-cm
2
cell culture fl ask (Corning cell culture fl asks; cat. no.
CLS3276).
11. CO
2
incubator (Thermo/Forma Scienti fi c CO
2
Water Jacketed
Incubators, Model 3121). All CO
2
incubators for cell culture
are suitable.
12. Luminescence microplate reader: Wallac Multilabel Counter
1420 (PerkinElmer, NL). Other microplate readers that can
measure bioluminescence are suitable.
13. 24-well (Corning; cat. no. CLS3524).
14. 96-well optical fl at-bottomed and black-framed microplates
(Nalge Nunc Intl.). All 96-well microplates with black frames
and clear fl at bottoms are suitable for luminescence measure-
ment (see Note 3).
15. Vortex shaker (Ika Labortechnik).
16. Infra-red heat lamp (home-made or from Science Products).
17. Biohazard Class II safety cabinet (see Note 4).
18. Table-top centrifuge (Beckman Coulter Allegra, GS-6 centri-
fuge). Most table-top centrifuges with a swing-out rotor are
suitable but these need to have a carrier assembly for plates
(Beckman Coulter; MicroPlus Carrier Assembly for GH-3.8
Rotors cat. no. BK362394).
19. Contura HS-40 shaver (Wella), used for removal of the fur
from the skin of mice by shaving (Optional).
2.4. Equipment

43330 Quantitative Analysis of Plasmodium berghei Liver Stages…
20. Anesthesia system (i.e., XGI-8 gas connected to the Lumina II
from Caliper) for anesthesia of mice prior to and during in vivo
imaging. Mice are anesthetized in the “induction chamber,”
which is pre- fi lled with the anesthetic vapor (iso fl uorane/air)
via the vaporizer unit, and are kept under anesthesia in the
imaging chamber by holding their muzzles close to a small
mask connected to the main vaporizer unit.
21. IVIS Lumina II System (Caliper Life Sciences, USA). All
in vivo imaging system with bioluminescent imaging option
are suitable for this purpose.
1. Imaging data are analyzed with the software provided with the
in vivo imaging system (i.e., LIVING IMAGE 4.1 for the
Lumina II from Caliper).
2. Microsoft Excel is used to conduct preliminary data analyses.
3. GraphPad Prism software (Graph-Pad software, Inc., USA) is
used for statistical analyses (best- fi t) effective concentration
(EC50) calculation.
This protocol requires the collection of Pb GFP-Luc
con
sporozo-
ites from infected A. stephensi mosquitoes. Sporozoites are
obtained from dissected salivary glands at days 20–28 after blood
feeding. Maintenance/rearing and infection of mosquitoes are
performed as described in (
13 ) . The total number of mosquitoes
required is dependent on the experiment and the number of sali-
vary gland sporozoites per dissected mosquito; in our laboratory
we usually obtain sporozoites loads of 0.5–1 × 10
5
per mosquito
(see Fig.
1 for a schematic representation of the work fl ow involv-
ing collection of sporozoites, in vitro culture and analysis of the
liver stages).
1. Transfer infected mosquitoes into a tissue net covered 50-ml
centrifuge tube.
2. Anesthetize mosquitoes with carbon dioxide (blown directly
into the tube). Keep the tube containing the anesthetized mos-
quitoes on ice until dissection.
3. Place the mosquito in one drop of PBS on a glass slide under a
stereomicroscope (magni fi cation 5–20×).
4. Carefully remove the mosquito head from thorax using a for-
ceps and an insulin needle. In general the two glands, each
with three lobes, will remain attached to the head (see Fig.
2 ),
2.5. Software
3. Methods
3.1. Analysis
and Quanti fi cation
of In Vitro Liver-Stage
Development
3.1.1. Collection
of Sporozoites
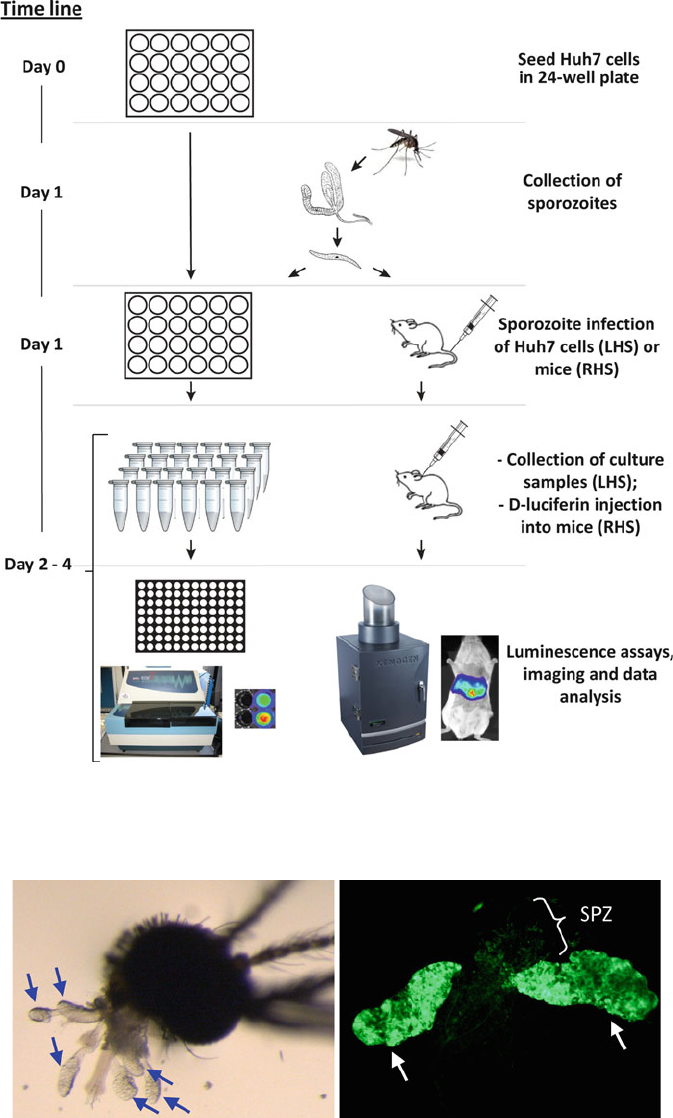
434 T. Annoura et al.
Fig. 1. Work fl ow scheme for quantitative analysis of Plasmodium liver stages. Left hand side (LHS): liver-stage development
in vitro analyzed by measuring luminescence using a plate reader. Right hand side (RHS): liver-stage development in vitro
analyzed by real-time bioluminescence imaging of live mice.
Fig. 2. ( a ) The view of a mosquito’s head attached to six intact salivary gland lobes ( grey arrows ; stereomicroscope; ..
magni fi cation). ( b ) GFP-expressing sporozoites of parasite line a Pb
GFP-Luc
con
in lobes of a salivary gland ( white arrows )
and free sporozoites (SPZ; fl uorescence microscope).
43530 Quantitative Analysis of Plasmodium berghei Liver Stages…
but sometimes they remain within the thorax. In this case,
gently press the forceps on the thorax in order to release the
salivary glands.
5. Collect and transfer the two salivary glands (with six intact
lobes) by forceps into a 1.5-ml Eppendorf tube containing
100 m l of incomplete RPMI1640 medium. It is important to
pick only the salivary glands without contamination with other
mosquito organs.
6. Repeat steps 4–6 until the required number of salivary glands
is collected.
7. In order to obtain free sporozoites from the salivary glands,
disrupt the collected salivary glands using a PP-pestle homog-
enizer and Vortex shaker (see Note 5).
8. In order to determine the total number of collected sporozo-
ites, take a 10 m l-sample (from the 100 m l) and place in a
Bürker-Türk cell counter.
9. First allow the sporozoites to settle before counting the
sporozoites by placing the cell counter for 20 min in a moist
chamber.
10. Sporozoite suspensions.
(a) For the in vitro assays, dilute the sporozoite suspension with
complete RPMI1640 medium (10% FBS), to a fi nal con-
centration of 0.3–10 × 10
5
sporozoites per 100 m l for trans-
fer to the Huh7 cultures (see Subheading
3.1.3 , step 2).
(b) For the in vivo assays, dilute the sporozoite suspension
with incomplete RPMI1640 medium to a fi nal concentra-
tion 1–200 × 10
3
sporozoites per 50–800 m l for injection
into mice (see Subheading
3.2.2 , step 4).
For principles of Huh7 cell culture, such as storage, thawing and
maintenance of cells, see the Japanese Collection of Research
Bioresources (JCRB) (
http://huh7.com/huh7_cell_culture.html ).
Most manipulations for the in vitro cultivation of Huh7 cells (in
combination with P. berghei parasites) are performed in a Class II
safety cabinet.
1. Defrost frozen stock-solution of 1 ml Huh7 cells (1 × 10
7
cells
in 1 ml of complete RPMI1640 medium, containing 10%
DMSO) in 10 ml Cell complete RPMI1640 culture medium
(20% FBS) into a 50-ml centrifuge tube.
2. Centrifuge at 13,000 × g for 10 min at RT using a tabletop
centrifuge and remove the supernatant.
3. Resuspend the cells by adding 30 ml of complete RPMI1640
culture medium (20% FBS) and seed into 75-cm
2
tissue culture
fl asks.
3.1.2. Culture of Huh7
Cells
436 T. Annoura et al.
4. Incubate the fl asks for 12–24 h in a CO
2
incubator (37°C,
5% CO
2
).
5. After this culture period, eliminate dead cells by removing
culture medium and washing the plates with 20 ml PBS. Add
30 ml complete RPMI1640 medium (20% FBS) and incubate
the plates for several days (see Note 6).
6. For collecting Huh7 cells for the “sporozoite assays,” remove
the culture medium from the plates and “detach” the Huh7
cells by adding 3 ml of the trypsin–EDTA 4Na solution for a
period of 5–10 min at 37°C.
7. To the (detached) cell suspension add 17 ml complete
RPMI1640 medium (10% FBS) and transfer to 50-ml tubes.
8. Spin at 200 × g for 10 min at RT in a table-top centrifuge,
remove the supernatant, and resuspend the cells in 10 ml com-
plete RPMI1640 medium (10% FBS).
9. Collect a sample of 10 m l for determination of the numbers of
Huh7 cells by counting in a Bürker-Türk counting chamber.
10. Adjust the volume of the Huh7 cell suspension with complete
RPMI1640 medium to obtain a fi nal concentration of 5 × 10
4
cells per ml.
11. Seed 5 × 10
4
Huh7 cells by adding 1 ml of the fi nal Huh7-cell
suspension to wells of a 24-well plate.
12. Before adding sporozoites to the wells, incubate the 24-well
plates for 12–24 h in a CO
2
incubator (37°C; 5% CO
2
). Huh7
cells will cover 40–60% of the well.
1. Remove the culture medium from the wells of the 24-well
plates (see Subheading
3.1.2 , steps 12–13) and subse-
quently add 900 m l fresh, pre-warmed (37°C) complete
RPMI1640 medium (10% FBS). See Note 7 for additional
information about the timing of adding inhibitors/drugs
to the cultures.
2. Add 100 m l of the sporozoite suspension containing 0.3–10 × 10
5
sporozoites (see Subheading
3.1.1 , step 10) to each well.
3. Spin at 13,000 × g for 5 min at RT (table-top centrifuge;
Beckman Coulter Allegra, GS-6 centrifuge with carrier assem-
bly for plates swing unit).
4. Incubate at 37°C and 5% CO
2
for 2–3 h.
5. Remove free sporozoites from the wells by removing the
medium and replacing it with 1 ml of fresh pre-warmed com-
plete RPMI1640 medium. See Note 7 for additional informa-
tion about the timing of adding inhibitors/drugs to the
cultures.
6. Return plates to the incubator at 37°C and 5% CO
2
.
3.1.3. Sporozoite Invasion
and Culture of the Liver
Stages in Huh7 Cells (with
or Without Addition of
Inhibitors)
43730 Quantitative Analysis of Plasmodium berghei Liver Stages…
Parasite liver-stage development in Huh7 hepatocytes is analyzed
over a 52-h period after the addition of sporozoites. After sporozo-
ite invasion, most P. berghei parasites develop in 60–64 h into
mature liver schizonts. After 64 h, merozoites are released from
the hepatocyte as merosomes, packets of 100–200 merozoites sur-
rounded by host cell membrane. Therefore, liver-stage develop-
ment can be quanti fi ed by measuring bioluminescence of lysed
cultured cells up to 52 h after sporozoite invasion, using a microplate
reader or by measuring bioluminescence directly in culture plates
using the IVIS Lumina II system (see Note 8). Usually, experi-
ments are performed in triplicate (3 culture wells per condition or
time-point).
1. Remove the culture medium from the wells of the 24-well
plates and add 1 ml PBS for washing.
2. Remove PBS and add 100 m l of 1× cell culture lysis reagent
(CCLR).
3. Mix the cells and the CCLR by pipetting until all cells are lysed
(when the bottom of the wells become clear and the lysis solu-
tion is homogenous).
4. Collect the cell lysis solution and transfer lysed cells from each
well to 1.5-ml Eppendorf tubes. These samples can be stored
at −80°C until ready to perform the luciferase assay.
5. When all samples have been collected and are ready for the
luminescence assay, add 100 m l of “luciferase assay substrate
solution” and 10 m l of the lysed cell samples into wells of a
black-framed 96-well plate. Samples containing uninfected
Huh7 cells are used as negative controls.
6. Measure the light reaction of each well for 10 s using a
microplate luminometer. The luciferase activities are expressed
as relative luminescence units (RLU) for each sample.
7. Export the data and proceed with statistical analysis of the
data.
This protocol requires Pb GFP-Luc
con
sporozoites collected from
salivary glands of infected A. stephensi mosquitoes at days 20–28
after blood feeding. Maintenance, rearing and infection of mosqui-
toes are performed as described in (
13 ) . The total number of mos-
quitoes required is dependent on the experiment and the number
of salivary gland sporozoites per dissected mosquito; in our labora-
tory we usually obtain sporozoites loads of 0.5–1 × 10
5
per mos-
quito (see Fig.
1 for a schematic representation of the work fl ow
involving collection of sporozoites, infection of mice with sporo-
zoites and analysis of liver stage development).
3.1.4. Quanti fi cation
of In Vitro Liver-Stage
Development (with or
Without Addition of
Inhibitors)
3.2. Analysis
and Quanti fi cation
of In Vivo Liver-Stage
Development
438 T. Annoura et al.
Sporozoites of the Pb GFP-Luc
con
line are obtained from dissected
salivary glands from infected mosquitoes as described in
Subheading
3.1.1 .
1. Place the mice under an IR heat lamp 5–10 min before injec-
tion of the sporozoites (see step 3). The tail veins swell at the
higher temperature, simplifying the intravenous injection pro-
cedure. In addition, prepare anesthesia system in suf fi cient
time, such as fi lling the “induction chamber” with the anes-
thetic vapor (iso fl uorane/air), to be able to inject the sporozo-
ites immediately after the puri fi cation procedure.
2. Prepare the sporozoite suspensions as described in
Subheading
3.1.1 , step 10.
3. Dilute the sporozoite suspension with incomplete RPMI1640
medium, to a fi nal concentration of 1–200 × 10
3
sporozoites
per 200 m l for injection into mice. This sporozoite suspension
is injected intravenously into the tail vein (see Note 9).
1. Prepare the in vivo imaging system for imaging the mice
(Fig.
3a shows the Lumina II and anesthesia system from
Caliper) (see Note 10).
2. For imaging liver stages, anesthetize infected mice at different
time points after sporozoite inoculation (e.g., 24, 40, 48 or
64 h) using the iso fl uorane-anesthesia system.
3. Remove the fur from the ventral part of the body by shaving
using a Contura HS-40 shaver. Removal of the fur is performed
to prevent quenching of the light signal, and must be done
carefully as a hematoma might in fl uence the imaging.
4. Inject 30 m l
D -luciferin substrate solution subcutaneously into
the neck of the anesthetized mouse (see Note 11).
5. Place the mouse on a piece of Art again paper and position it
under the camera in the centre of the sample stage (if needed,
fi x with black tape). The gated sample stage is pre-warmed to
37°C and thereby stabilizes the body temperature of the mouse
(see Note 12).
6. Wait for 3 min before acquiring the bioluminescence image.
This period allows circulation of the D- luciferin substrate
within the body of the mouse.
7. Acquire the bioluminescent image. The bioluminescent signal
collected is linearly related to the exposure time within a range
of 5 s to 10 min. Routinely, we image infected mice for
60–180 s when mice have been injected intravenously with
1 × 10
4
sporozoites. If necessary, acquire a new image with
shorter or longer exposure time.
3.2.1. Preparation of
Sporozoites
3.2.2. Infection of Mice
with Sporozoites
3.2.3. Quanti fi cation of
Liver-Stage Development
In Vivo (with or Without
Drug Treatment of Mice)

43930 Quantitative Analysis of Plasmodium berghei Liver Stages…
8. After exposure is complete, the overlay of the photographic
and luminescent picture is displayed. See Fig.
3b for represen-
tative images of luminescent signals of liver stages in mice
infected with Pb GFP-Luc
con
sporozoites at different time points
after infection.
9. Save imaging data for post-processing analysis (i.e., measure-
ment of the intensity of bioluminescent signals in a speci fi c
area (see Subheading
3.2.4 )).
10. Remove the mouse from the imaging chamber and repeat steps
3–8 for a new mouse.
Fig. 3. ( a ) As an example, the in vivo imaging system “IVIS Lumina II” from Caliper is shown, which has been used in our
laboratory for real-time imaging of P. berghei liver stages in whole bodies of live mice and in isolated, non fi xed livers.
( b ) In vivo images of the same mouse with a developing liver-stage infection, at different time points after intravenous
inoculation of 10,000 Pb GFP-Luc
con
sporozoites. At 64 h, merozoites are released from the liver into the blood circulation.
The relative luminescence units (RLU), at each time point, are shown under the picture of the mouse.

440 T. Annoura et al.
The whole-body bioluminescence images of mice provide a
qualitative assessment of the load/intensity of Pb GFP-Luc
con
liver
stages within an animal and this liver load can be directly compared
between different animals if the same measurement settings during
the experiments are maintained. Most in vivo imaging systems
software contain tool options that enable the quanti fi cation of bio-
luminescent signal emanating from speci fi c areas of the mouse
(“region of interest,” ROI).
1. Select the image to analyze.
2. Create region of interest (ROI; Liver) on the image (see
Fig.
3b ).
3. Determine the bioluminescent intensity in ROI. The measure-
ment of the signals results in the generation of a “measurement
table” that contains data on the ROI measurement (total and
average photon counts) and ROI information (dimension,
size, etc.).
4. Export the data and eventually proceed with statistical analysis
(see Note 13).
1. All experiments using mice must be performed according to
the applicable national guidelines and regulations. Diets of
laboratory rodents with low content of total protein, energy,
and/or p -aminobenzoic acid (PABA) can negatively in fl uence
P. berghei infections (
14 ) . In our laboratory, we therefore pro-
vide diets with high protein content (20–25% of total and gross
energy content; 18,000–20,000 kJ/kg). If transgenic mice are
used, they should not express luciferase gene(s) that use
D -
luciferin as a substrate. Mice are kept under normal (day/
night) light conditions.
2. It is very important to dissolve inhibitors/antimalarial drugs
completely; vortexing and/or sonication and/or 37°C incu-
bation can help to dissolve compounds. The inhibitors in
stock solution can be diluted with DMSO or culture medium
according to their properties. It is better to make serial dilu-
tions fresh, though they can also be stored at −20 or −80°C.
We prepare stock solutions at a 100 times the fi nal concentra-
tion required in the well so that when the drug is added to the
well the concentration of DMSO in overnight culture is <1%,
which is not harmful to the parasite development. No inhibi-
tion controls (i.e., culture without inhibitors) also contain
<1% DMSO.
3.2.4. Image Analysis
4. Notes
44130 Quantitative Analysis of Plasmodium berghei Liver Stages…
3. For luminescence measurements, we routinely use black-framed
microplates as they best reduce light scattering between wells,
which can arti fi cially increase the signals detected in neighboring
wells and thereby calculated EC50 values.
4. Most manipulations with Huh7 cells and with genetically
modi fi ed P. berghei parasites are performed in a Class II safety
cabinet.
5. It is important to maximize the release of sporozoites from the
dissected salivary glands. However, one should not homoge-
nize the salivary gland sample too much as this can reduce the
number of sporozoites. We therefore do the following: 10 s
vortexing, ten strokes of the homogenizer, 10 s vortexing.
6. Usually we check the plates regularly (once a day) under the
inverted microscope (10–20× magni fi cation) for the cell
growth in these plates. Huh7 cells are usually collected for the
sporozoite assays when cells are 70–80% con fl uent.
7. If you aim to analyze the effect of inhibitors on sporozoite
invasion, inhibitors can be added to the 24-well culture plates
during this step. If you aim at analyzing the effect speci fi cally
on the development of the liver stages (after invasion of hepa-
tocytes by the sporozoites) inhibitors should be added during
Subheading
3.1.3 , step 5. See also Note 3 for more details on
adding inhibitors to the cultures.
8. Liver-stage development can be quanti fi ed by measuring bio-
luminescence in lysed culture samples using a microplate reader
(see Subheading
3.1.4 , steps 1–7) or by measuring biolumi-
nescence directly in culture plates using the IVIS Lumina II
system. For direct imaging we directly add
D -Luciferin (80 mg/
ml) to the wells wait for 3 min and image the plates in the IVIS
Lumina II system in a comparable way as described for imag-
ing infected mice (see Subheading
3.2.3 , steps 7–9).
9. Usually mice are infected intravenously with de fi ned numbers
(i.e., 10
2
to 10
6
) of freshly dissected sporozoites. However,
other studies may require sporozoites be administered by other
routes of administration. For intradermal inoculation we inject
50–100 m l (with varying numbers of parasites) of the sporozo-
ite suspension into the skin at various site of the body (most
often the neck). For subcutaneous inoculation we usually inject
500–800 m l (and varying numbers of parasites) of the sporozo-
ite suspension in the scruff of the neck.
10. Use the in vivo imaging system as recommended by the manu-
facturer. Ensure that the system is operational and that the
automatic background measurements have been performed
with settings that will be used for imaging the mice. Systems
are run by speci fi c software that also serves as a guide to help

442 T. Annoura et al.
(inexperienced) users through the steps associated with
quantitative in vivo imaging and data analysis.
11. Reproducible imaging results are obtained with subcutaneous
injection of the substrate. However, we have evidence that
intravenous injection of
D - luciferin might improve the sensitiv-
ity of imaging, especially for organs that could degrade/elimi-
nate the substrate more rapidly or are less accessible for the
substrate.
12. Ensure that the fi eld of view is set to provide an imaging area
that is wide enough to encompass the entire sample or the area
of interest. Distances of 10 cm are used for imaging a whole
body. Remove all dust particles from the sample stage. Mice
can be fi xed by taping the legs to prevent them from moving
and interfering with the imaging of organs; black tape should
be used for fi xing the mice to prevent background light
emission.
13. Bioluminescence imaging is simple to execute, allows monitor-
ing of the course of biological processes without killing the
experimental animal, and therefore reducing the number of
animals required for experimentation because multiple mea-
surements can be made in the same animal over time, minimiz-
ing the effects of biological variation. Usually, we use the mean
luminescent values of bodies/organs of three mice per imaging
time point.
Acknowledgment
TA, SC, and SMK received fi nancial support of Top Institute
Pharma (The Netherlands) project: T4-102.
References
1. Bruna-Romero O et al (2001) Detection of
malaria liver-stages in mice infected through
the bite of a single Anopheles mosquito using a
highly sensitive real-time PCR. Int J Parasitol
31:1499–1502
2. Siau A et al (2008) Temperature shift and host
cell contact up-regulate sporozoite expression of
Plasmodium falciparum genes involved in hepa-
tocyte infection. PLoS Pathog 4:e1000121
3. Li J et al (1991) Plasmodium berghei : quantita-
tion of in vitro effects of antimalarial drugs on
exoerythrocytic development by a ribosomal
RNA probe. Exp Parasitol 72:450–458
4. Hobbs CV et al (2009) HIV protease inhibitors
inhibit the development of preerythrocytic-stage
Plasmodium parasites. J Infect Dis
199:134–141
5. Cunha-Rodrigues M et al (2008) Genistein-
supplemented diet decreases malaria liver infec-
tion in mice and constitutes a potential
prophylactic strategy. PLoS One 3:e2732
6. Fisk TL et al (1989) In vitro activity of antima-
larial compounds on the exoerythrocytic stages
of Plasmodium cynomolgi and P. knowlesi . Am J
Trop Med Hyg 40:235–239
7. Scho fi eld L et al (1987) Interferon-gamma inhib-
its the intrahepatocytic development of malaria
parasites in vitro . J Immunol 139:2020–2025
8. Gego A et al (2006) New approach for high-
throughput screening of drug activity on
44330 Quantitative Analysis of Plasmodium berghei Liver Stages…
Plasmodium liver stages. Antimicrob Agents
Chemother 50:1586–1589
9. Ploemen IH et al (2009) Visualisation and
quantitative analysis of the rodent malaria liver
stage by real time imaging. PLoS One 4:e7881
10. Mwakingwe A et al (2009) Noninvasive real-
time monitoring of liver-stage development of
bioluminescent Plasmodium parasites. J Infect
Dis 200:1470–1478
11. Portugal S et al (2011) Host-mediated regula-
tion of superinfection in malaria. Nat Med
17:732–737
12. Franke-Fayard B et al (2004) A Plasmodium
berghei reference line that constitutively
expresses GFP at a high level throughout the
complete life cycle. Mol Biochem Parasitol
137:23–33
13. Sinden RE (1997) Infection of mosquitoes
with rodent malaria. in The molecular biology
of insect disease vectors. eds Crampton J. M.,
Beard C. B., Louis C. (Chapman & Hall,
London, United Kingdom), pp 261–267.
14. Gilks CF et al (1989) Host diet in experimental
rodent malaria: a variable which can compro-
mise experimental design and interpretation.
Parasitology 98:175–177
