
Behavioral Characters for the Higher Classification of Orb-Weaving Spiders
Author(s): William G. Eberhard
Source:
Evolution,
Vol. 36, No. 5 (Sep., 1982), pp. 1067-1095
Published by: Society for the Study of Evolution
Stable URL: http://www.jstor.org/stable/2408084 .
Accessed: 11/02/2011 13:36
Your use of the JSTOR archive indicates your acceptance of JSTOR's Terms and Conditions of Use, available at .
http://www.jstor.org/page/info/about/policies/terms.jsp. JSTOR's Terms and Conditions of Use provides, in part, that unless
you have obtained prior permission, you may not download an entire issue of a journal or multiple copies of articles, and you
may use content in the JSTOR archive only for your personal, non-commercial use.
Please contact the publisher regarding any further use of this work. Publisher contact information may be obtained at .
http://www.jstor.org/action/showPublisher?publisherCode=ssevol. .
Each copy of any part of a JSTOR transmission must contain the same copyright notice that appears on the screen or printed
page of such transmission.
JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of
content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms
of scholarship. For more information about JSTOR, please contact [email protected].
Society for the Study of Evolution is collaborating with JSTOR to digitize, preserve and extend access to
Evolution.
http://www.jstor.org

Evolution, 36(5), 1982,
pp.
1067-1095
BEHAVIORAL
CHARACTERS FOR THE
HIGHER CLASSIFICATION OF
ORB-WEAVING SPIDERS
WILLIAM
G.
EBERHARD
Smithsonian
Tropical
Research Institute
and
Escuela de
Biologia,
Universidad
de Costa
Rica,
Ciudad
Universitaria,
Costa
Rica
Received August 25, 1980. Revised June
22, 1981
A number
of studies
have
shown
that
behavior
patterns
can be useful
taxonomic
characters
(see
review
by
Mayr,
1958; also
Evans,
1966;
Crane,
1975;
Michener
et
al.,
1978;
Eickwort
and
Sakagami,
1979;
Greene,
1979).
The webs
and web-build-
ing
behavior
of
orb-weaving
spiders
are
complex,
apparently
stereotyped
charac-
ters,
and as such
offer
promise
of
being
useful
in
indicating
taxonomic
affinities.
As
Levi (1978a,
1978b) has
noted,
how-
ever,
this
promise
has not
been fulfilled.
The
gross,
relatively
superficial
web
char-
acters such
as the
presence
or absence
of
stabilimenta,
or
open
versus closed
hubs
which have been
studied
to date have not
proved
to
be useful
indicators of
subfa-
milial relations.
Webs are
directly
in-
volved
in
orb weavers' interactions
with
a number
of
aspects
of their environments
(particularly
prey),
and
relatively
minor
changes
in
environmental
factors
could
result
in selection
for changes
in
web
form.
Levi
argues
(echoing
Darwin,
1859)
that
at least some
aspects
of web
design
might
thus
be
expected
to be evolutionarily
non-
conservative
and
of little
use
in
indicating
higher
taxonomic
relationships.
This does
not,
however,
eliminate
the
possibility
that webs and web-building
behavior
may
be
useful
as taxonomic
characters.
It
is
possible
that some
details
of web
design
with
apparently
low
func-
tional significance
may
be
more conser-
vative
than other
more
obviously
func-
tional characters.
I
have
the
impression
that
many
web
characters
are
not scat-
tered randomly
among
the
webs
of ara-
neoid
species,
and that their patterns
of
occurrence will
be
of at least some use
in
systematics,
particularly
at
generic
and
tribal levels.
The
analysis
necessary
to
substantiate this has
yet to be performed,
although Risch (1977)
has
made a
prom-
ising start by finding
that two congeneric
species' webs were
more similar to each
other in
a number of
details
than to those
of two species
from
other genera.
It is also possible
that some details of
construction behavior
are
employed by
spiders
in
unchanged
form to construct
a
variety
of different web forms,
and that
these
behaviors
are
thus slow
to evolve
even while
the
webs themselves change
rapidly. This paper
describes several such
behaviors and shows that they are conser-
vative
enough
to
characterize
the classical
subfamily
and
family groupings
which
have been
based on
adult morphology
and
can
thus
be
used
to indicate relationships
between
them,
a topic on which there is
currently substantial
disagreement (e.g.,
Lehtinen, 1967, 1975;
Levi, 1978a, 1980;
Robinson and Robinson,
1978, 1980;
Opell, 1979).
Obviously
the more characters used
in
constructing
a
system
of
relationships
the
greater the likelihood
of the results being
correct.
A
great deal
is
known about the
morphology of orb
weavers, but no com-
prehensive surveys
of characters are yet
available
(but
see
Levi, 1980),
so
they
can-
not yet be included.
This paper is meant
to provide
useful
data
for
later syntheses,
not to give the final
word on the classifi-
cation of
orb
weavers.
Choice
of
Characters
When
one has
collected data
on
a
pre-
viously
untried character or set of char-
acters,
the first
step
in
their
analysis
is to
compare
their
distribution
with
previous
taxonomic
schemes based on other char-
acters.
If
the new
characters
are function-
1067

1068
WILLIAM
G.
EBERHARD
ally
independent of
the older
ones-as
would
appear to be the
case here since
the
morphology of male
and female
genitalia,
eye
positions and
structures,
cheliceral
morphology,
cephalothorax form and
oth-
er
morphological
characters used by
other
workers
would seem to
have
little func-
tional
relation to the
details
of
leg move-
ments
and thread
manipulation
during
web
construction and
attack and
court-
ship
behavior-then there
are
several
pos-
sible
results.
If
the
previous schemes are
all
incorrect,
the
distribution of the
new
characters will not be
in
accord with
any
of
them,
and the new
characters can be
used to construct a new
set
of
relation-
ships.
If,
on
the other
hand, the previous
schemes
are at
least
approximately
cor-
rect,
then the degree
to which the
new
characters "fit" will
depend
on
the rates
of
evolution of these
characters. Those
which
have evolved
very slowly will be
uniform
over
many
different
groups,
those
which
have evolved
relatively rapidly
will
vary
even
within given
taxa,
and still oth-
ers will more or
less match the taxonomic
scheme. Since some of the
behavioral
characters
examined
in
this
study
do have
distributions similar
to
the
classical
group-
ings of
Simon (1892)
based on adult
mor-
phology, this scheme
is probably at
least
approximately
correct.
There were other
characters
which
were
constant
in
all
the
groups
studied
(e.g.,
starting sticky spiral
from the
edge
rather
than
the
center
or
any
other
part
of the
web),
and
others which varied within
giv-
en
subfamilies
(e.g.,
pulling
motions of
legs
IV on
sticky spiral
as
it
was pro-
duced). These behaviors are not included
here since the basic
objective of the
study
was,
after
testing
the
classical
scheme,
to
attempt to use the behavioral
characters
to
indicate
relationships
between
subfam-
ilies and families. Thus
only those char-
acters which
appear
to be
constant
or
nearly
constant
within
subfamilies or fam-
ilies and which
also
differ
between them
are
discussed.
Detailed
descriptions
of
these
characters,
the different states which
they
assume,
their
functional indepen-
dence,
and
the
most
probable
directions
of
transformation
are
given
in
Appen-
dix
1.
MATERIALS
AND
METHODS
Specimens
of
spiders
are
deposited
in
the
Museum
of
Comparative
Zoology,
Cambridge,
Mass.
02138.
The
specimen
numbers
mentioned
in
the
text
and
ap-
pendices
refer to
numbered
labels
includ-
ed
in
individual
spiders'
vials. At
present
it is
unfortunately
impossible to
identify
many
(most?)
orb-weaving
spiders
at
the
species
level, and
most
specimens
are
giv-
en
only
generic
names.
This situation
does
not
seem
likely
to
change
radically in
the
near
future,
and
it
thus
seemed
wise
to
proceed
with
the
presentation
of
the
data
in
this
paper
rather
than
wait for
more
complete
identifications.
Unless
otherwise
noted all
references
to
subfamilial
and
tribal
groupings are
based on
the monu-
mental
work
of
Simon
(1892).
The
ulob-
orid
names follow
Opell
(1979).
The
techniques
of
observation
and
their
limitations
are
described in
the
Appendi-
ces.
As
might
be
anticipated,
many
details
of
web-building behavior are
extremely
stereotyped
within
a
given
species
(see
Appendix
1).
This
uniformity
makes
web-
building
behavior
an
attractive
set of
characters
to
study because
relatively brief
observations
suffice to
characterize
a
species.
RESULTS AND
DISCUSSION
Observations of
at least
148
species
in
at
least
55
genera
are
presented
in
Appen-
dices
2
and
3
and
summarized
in
Table 1.
The
data
are
arranged
according to
the
groupings
of Simon
(1892). Both
substan-
tial
concordance within
subfamily
and
family
groups
with
respect
to a
number
of
characters and
clear
differences
between
subfamilies and
families are
evident.
These
patterns constitute
confirmation of
the
classical
groupings.
The one
distinction
which
is
not
confirmed is
that
between
Tetragnathinae and
Metinae,
as there
were
no
consistent
differences between
species
of
these
two
groups.

SPIDER BEHAVIOR AND
TAXONOMY
1069
TABLE 1. Summary of data in
Appendices
2
and
3
and Robinson
and Robinson, 1980. The data are
relatively
scarce for some characters for
Theridiosomatidae
and
Anapidae
and their characterization here is
tentative.
Parentheses indicate character
states thought to
be
secondarily
derived within the group;
in
all cases
fewer
than 10% of the species observed
for that group have the presumed
secondary
state,
and the species
involved
have all been
classified
without
question on morphological grounds
in the taxa in which they are placed
here.
Further
justifications
for considering
these as convergences rather
than synapomorphies are given in
the text
and Appendices
2
and
3. The numbers of
the
character states
refer to designations
in
Appendix
1 and the
text.
Behavior
Group
A B C D E
F G H I J
Araneinae
2
(4,
"3") 1
1 2
(1, 3)
1 1
(3, 4)
2
&
3
(1)
1
(3)
3
& it
2
(1)
Tetragnathinae-
Metinae
1
(2)
1
1 1
(3)
1
1 2
(1, 3)
1
(3)
2
?1tt
Nephilinae
3
1 1 1 & 2
1
2
1 1 1 1
Theridiosomatidae-
Anapidae
1
& 4
1
1 1 & 3
1
1
4
&
5
2
& 3
1 & 2 ?
Uloboridae
2
2 2 1 & 2 2
(1)ttt
4 1 1 2
?
t
Gasteracantheae, Micratheneae,
Mastophoreae, Cyrtarachneae,
and Celaenieae
only.
tt
Pers.
observ.
of
Leucauge sp
near venusta
and
Tetragnatha sp. (#0-19-1);
TR
also absent
in
Tetragnatha spp.
and
Pachygnatha
spp.-
see Bristowe, 1958
ttt Hyptiotes spp.
which
spin
reduced, presumably
derived orbs
(e.g., Marples
and
Marples, 1937; Comstock, 1940).
Relationship
between
Uloboridae
and
Araneoid
Orb Weavers
There
has
been
a long
unresolved
con-
troversy
concerning
the possibility
that
orb web
construction,
which
is known
in
six
different
spider
families,
evolved more
than once
(see
Kaston,
1964,
and
Kull-
mann,
1972,
for the
most recent
summa-
ries).
There
is general
agreement
that
the
five araneoid
orb-weaving
families (Ara-
neidae,
Theridiosomatidae,
Anapidae,
Mysmenidae,
and
Symphytognathidae
sensu
Forster
and
Platnick,
1977)
all
evolved
from a single
ancestor,
but
their
relationship
to the
sixth
family,
Ulobori-
dae, is
disputed.
Some
authors
have
thought
that
the
lack
of
clear
synapomor-
phies
shared
by
Uloboridae
and
other,
non-orb-weaving
taxa
plus
the
similarities
between
the
designs
of the
webs
and the
web-building
behavior
of uloborids
and
araneoids
are
so great
as
to make a
com-
mon derivation
inescapable
(e.g.,
Pe-
trunkevitch,
1926;
Wiehle,
1928;
Lehti-
nen,
1967;
Opell,
1979;
Levi,
1980b;
see
also
Brignoli,
1979).
But
in
other,
more
widely
used schemes
(e.g.,
Simon,
1892)
uloborids
are
widely
separated
from
ara-
neoids on the basis of their possession of
a pair
of
structures (cribellum and cal-
amistrum) involved in the production of
one type
of
sticky
silk. The
presence
or
absence
of these structures has
recently
been shown, however,
to be
unreliable
as
an
indicator
of
higher-level relationships
in
several other spider groups (e.g., Lehti-
nen, 1967; Forster, 1970; Kullmann and
Zimmermann, 1976).
The distributions of behavior patterns
in
Table
1
support the idea that Araneidae
is more closely related to Theridiosoma-
tidae
and Anapidae than to Uloboridae
(fragmentary data on Mysmenidae and
Symphytognathidae will be presented
elsewhere;
they suggest
close
ties between
these
families and Anapidae). They do
not
permit
one
to
decide whether
or
not all
four
groups
evolved
from
a common orb-
weaving ancestor,
but
they suggest that
they
did
not.
In
order
to
decide between
a
single
or a dual
origin
of
orb webs,
one
must compare the orb weavers' behavior
with
comparable behavior of non-orb-
weaving
araneoid
groups
such
as
therid-
iids (possible sister groups of araneoid orb
weavers) and non-orb-weaving cribellates
such as
Dictynidae (possible
sister
groups

1070
WILLIAM
G.
EBERHARD
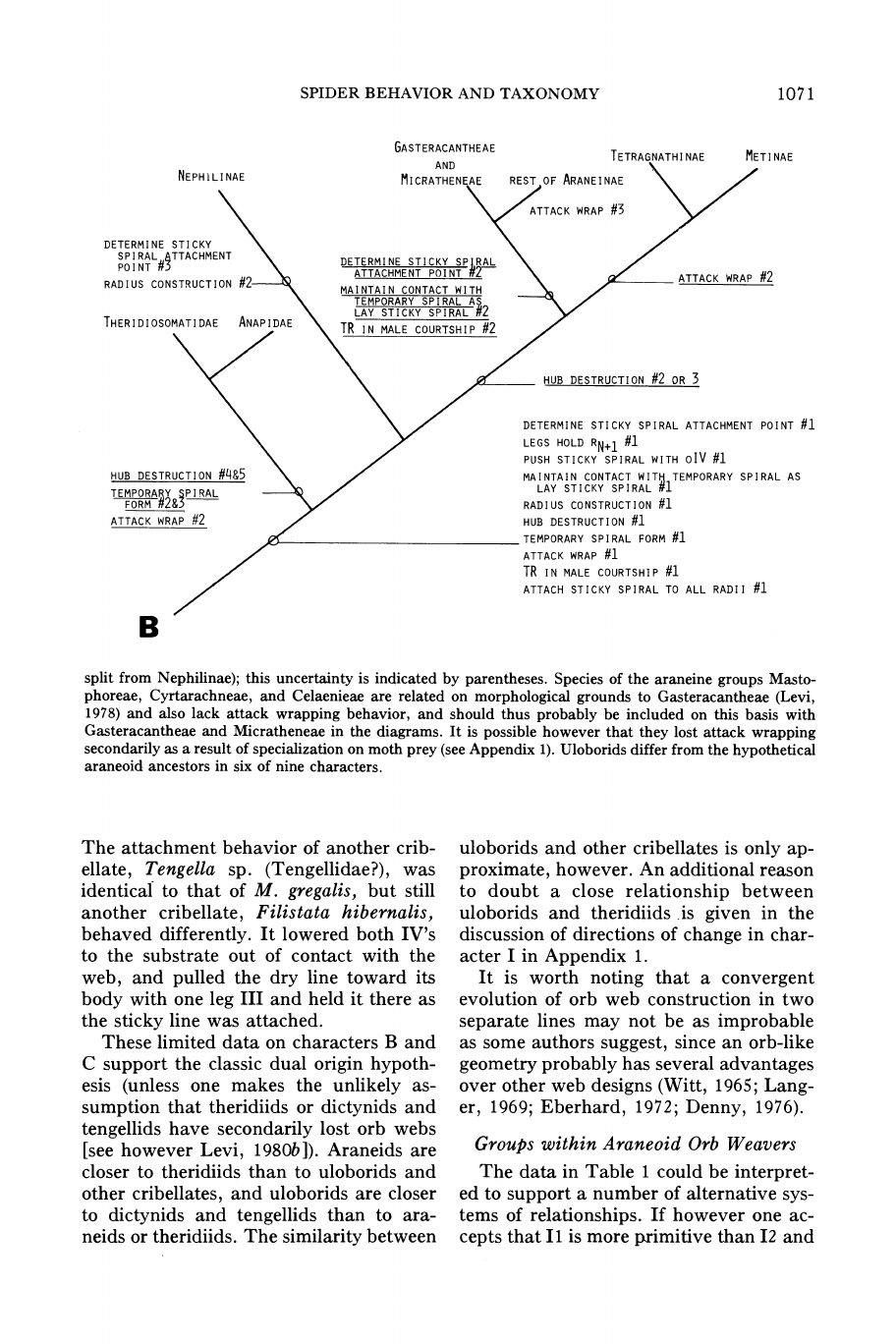
THERIDIOSOMATIDAE
ANAPIDAE
TETRAGNATH
I
NAE
METINAE
GASTERACANTHEAE
AND
REST
OF
MICRATHENEAE
ARANEINAE
HUB
DESTRUCTION
#4&5
ATTACK
WRAP
#3 TEMPOR
&PIRAL
DETERMINE
STICKY
SPIRLL
ATTACHMENT
POINT
#2
MAINTAIN
CONTACT
WITH
TEMPORARY
SPIRAL A$
LAY
STICKY
SPIRAL
I2\
ATTACK
WRAP
#2
TR IN
MALE
COURTSHIP
#2
NEPHI
L
INAE/
DETERMINE
STICKY
SP#3AL
HUB
DESTRUCTION
#2 OR
3
ATTACH
STICKY
SPIRAL
ALL
RADII
ATTACHMENT
POINT
(DTRIESTCYSIA
RADIUS
CONSTRUCTION
#2
ATTACHMENT
POINT
LEGS
HOLD
RN+1
#1
PUSH
STICKY
SPIRAL
WITH
oIV
#1
MAINTAIN
CONTACT
WITH
TEMPORARY
/ ~~~~~~~~~~~~~SPIRAL
AS LAY
STICKY
SPIRAL
#
/ ~~~~~~~~~~~~~(RADIUS
CONSTRUCTION
#1)
/ ~~~~~~~~~~~~~~(HUB
DESTRUCTION
#1)
TEMPORARY
SPIRAL
FORM
#1
A
ATTACK
WRAP
#1
TR IN
MALE
COURTSHIP
#1
FIG.
1.
Schemes
of
relationships
between
araneoid
orb-weavers
favored
by
the
data
in
Table
1.
A)
Most
parsimonious
scheme
assuming
that
lack
of
attack
wrapping
(I1)
is primitive.
B)
Alternative
scheme
assuming
that
attack
wrapping
evolved
independently
in
Theridiosomatidae-Anapidae.
Proposed
synapomorphies
are
underlined,
autapomorphies
are
not.
The
placement
of male
courtship
behavior
is
tentative
since
no
data
are
available
for
some
groups.
The
probable
character
states
of the
ancestral
stock
are
given
for all
characters.
There
is
less
certainty
for some
of
these
than
others
(e.g.,
the
character
states
designated
as
autapomorphic
in A
for
Nephilinae
could
also
be plesiomorphies
and
the
alternative
states
synapomorphies
in the
line
which
of
Uloboridae).
Since
the behaviors ex-
amined here are
mostly
involved
with
orb
web
construction,
this is not
strictly pos-
sible. However some
non-orb weavers
do
attach
sticky
lines to
non-sticky lines, and
the positions of
their legs
III
and IV can
be compared with
characters
B
and C of
the orb weavers.
The following observa-
tions were made
in
an attempt to permit
such
comparisons.
The
mesh-weaving araneoid Achaear-
anea
tepidariorum
(Theridiidae) attached
a line with
sticky silk to a dry line with
the same movements
as those
of
all ara-
neoid
orb-weavers (ipsilateral legs
III
and
IV held the dry
line on either side of the
attachment,
and
the other
leg
IV held the
sticky line), thus
suggesting
a closer rela-
tion of araneoid orb
weavers to Theridi-
idae than
to
Uloboridae.
The
cribellate
mesh-weaver
Mallos gregalis
(Dictyni-
dae), on
the other hand,
did not
use either
of the
hind legs to
stretch -the
cribellum
silk as
it was attached,
thus
resembling
the uloborids.
Both
legs IV seized
the dry
silk
(probably
more than one line-I
could
not convince
myself
on this point),
but in-
stead of holding
one IV anterior to the
oth-
er, they
were both equally
to
the side and
posterior
to
the
spinnerets,
and these
were
then touched
closer
to
one
leg
and some-
what anterior to
it. Thus this species'
be-
havior
was similar
but clearly
not identi-
cal to that
of the
uloborids.
Another
unidentified
dictynid
species was
so small
and
moved
so quickly
that the
details of
its behavior
could
not be distinguished,
but
it
seemed
to behave as
did
M.
gregalis.
SPIDER BEHAVIOR AND TAXONOMY 1071
GASTERACANTHEAE
TETRAGNATHI
NAE METI NAE
AND
NEPH1LINAE
MICRATHENEAE
REST OF
ARANEINAE
\
\f/T~~~~~~~~~TTAC
K
WRAP
#3
\
DETERMINE
STICKY
SPIRAL#f
TACHMENTDEEMNSTCYPRA
POINT
ATTACHMENT
POINT 2
\
ATTACK WRAP #2
RADIUS
CONSTRUCTION
#2
MAINTAIN
CONTACT WITH
\TEMPORARY
SP
IRAL
AS
\ LAY
STICKY
SPIRAL
2
THERIDIOSOMATIDAE
ANAPIDAE
TR IN MALE COURTSHIP #2
K
\ /< ~~~~~~~~~HUB DESTRUCTI
ON
#2
OR
3
DETERMINE
STICKY SPIRAL
ATTACHMENT POINT
#1
\
/ ~~~~~~~~~LEGS
HOLD
R
N+1
#1
\
/
~~~~~~~~PUSH
STICKY
SPIRAL WITH
oIV #1
HUB DESTRUCTION
#4'5
MAINTAIN CONTACT
WI%TM
TEMPORARY
SPIRAL
AS
TEMPORARY
,PIRAL
LAY STICKY SPIRAL
FTORM
#2&
RADI US
CONSTRUCTION
#1
ATTACK WRAP
#2
/HUB DESTRUCTION
#1
FOR
TEMPORARY SPIRAL
FORM
#1
K
2ATTACK
WRAP
#1
/ ~~~~~~~~~~~TR
I N MALE COURTSH IP
#1
ATTACH
STICKY SPIRAL TO ALL RADII #1
B
split
from
Nephilinae);
this uncertainty
is indicated by parentheses.
Species of the araneine
groups
Masto-
phoreae, Cyrtarachneae,
and Celaenieae
are related on morphological
grounds to
Gasteracantheae (Levi,
1978) and also lack
attack wrapping behavior,
and should thus
probably be included
on this basis with
Gasteracantheae
and Micratheneae in the
diagrams. It is possible
however that they
lost attack wrapping
secondarily
as a result of specialization on
moth prey (see Appendix
1). Uloborids differ
from the hypothetical
araneoid ancestors
in
six
of
nine characters.
The attachment behavior of another
crib-
ellate, Tengella sp. (Tengellidae?),
was
identical
to that of
M.
gregalis,
but still
another cribellate,
Filistata
hibernalis,
behaved differently. It lowered both
IV's
to the substrate out of contact
with the
web, and pulled the dry line toward
its
body with one leg
III
and held it
there as
the sticky line was attached.
These limited data on characters
B
and
C
support the classic
dual
origin
hypoth-
esis (unless one makes
the
unlikely
as-
sumption
that theridiids or
dictynids
and
tengellids have secondarily lost
orb webs
[see however Levi,
1980b]).
Araneids are
closer to theridiids than to uloborids and
other cribellates,
and uloborids are closer
to dictynids and tengellids
than to ara-
neids or theridiids. The similarity
between
uloborids
and other
cribellates is only ap-
proximate, however. An
additional reason
to
doubt a close
relationship between
uloborids
and theridiids is
given
in
the
discussion of directions of
change
in
char-
acter
I in
Appendix
1.
It is
worth noting that
a convergent
evolution
of orb web construction
in
two
separate
lines may not be as
improbable
as some authors
suggest,
since an
orb-like
geometry
probably has several
advantages
over
other
web
designs
(Witt, 1965; Lang-
er, 1969;
Eberhard, 1972;
Denny, 1976).
Groups
within Araneoid Orb Weavers
The
data
in
Table
1
could be
interpret-
ed to
support
a
number
of alternative
sys-
tems of
relationships.
If
however one ac-
cepts that 11 is more
primitive
than
12
and

1072
WILLIAM
G.
EBERHARD
13
and that
only in
well-defined
conditions
will a
reversion
occur (see
Appendix
1),
much of
the
ambiguity
disappears. The
system
of
relationships illustrated
in
Fig.
la
seems to
be
favored. It
should be
noted
that
the lack
of
attack
wrapping
(I1) in
Gasteracantheae and
Micratheneae,
which
nevertheless
show
three
characters
which
may
be
synapomorphies
with the
rest of
the
araneines
(Al,
D2, and
J2)
necessitates
an
independent
evolution of
attack
wrap-
ping
in the
groups
"other
Araneinae" and
Tetragnathinae-Metinae.
Postulation of
such
a
convergence
is justified
by
three
considerations: 1)
convergence has
already
been
documented
for
post-attack
wrap-
ping
behavior
in
at
least
four
groups
of
spiders
(Eberhard,
1967;
Rovner
and
Knost, 1974;
Robinson and
Lubin,
1979);
2)
convergence has
also
occurred
in
im-
mobilization
wrapping in
such
diverse
groups as
Pholcidae,
Hersiliidae,
Oecobi-
idae, and
Theridiidae (pers.
observ.); and
3) the details of
araneine attack
wrapping
seem to be
consistently
different
(13)
from
those of
other araneids
(12)
(see
Appendix
3).
The same
general
argument
favoring
independent
evolution
of
attack
wrapping
could also
be applied
to
wrapping
attacks
by
Theridiosomatidae
(still
poorly docu-
mented),
and this
could permit
a
system
of
relationships (Fig.
lb)
more
in
accord
with
other schemes
of
relationships
that
have
been
proposed
on
the basis
of
adult
morphology
and
which is
thus
probably
more
likely
to be
correct.
It
is
interesting
to note that
the
char-
acter
A3
which is
characteristic of Ne-
philinae can be
performed
only
in
webs
in
which
the
separation
between radii is
small
compared to the
span
of
the
spider's
legs,
and
is also
energetically
the least
costly
since the
spider
moves
directly
from
one
attachment to the next
(see
Peakall and
Witt,
1976,
for a
preliminary
discussion
of
the costs of
movements
during
web con-
struction).
This
suggests
that
the
evolution
of
A3
behavior
may
have
occurred
at
the
same
time as or
after the
development
of
tightly
meshed
webs.
All
of the few
known
nephiline
webs-Nephila
madagascaren-
sis
(Wiehle,
1931),
N.
clavipes,
N.
macu-
lata
(Robinson
and
Robinson,
1973),
Her-
ennia
ornatissima
(Robinson
and
Lubin,
1979a),
and
Nephilengys
malabarensis
(pers.
observ.-are
indeed
tightly
meshed,
and
the
Micrathena
species
(gracilis
and
#2200)
which
have
apparently
conver-
gently
evolved A3
behavior
also
make rel-
atively
tightly
meshed
webs and
only
use
this
behavior
in
the
central
portion
where
the
mesh
is
smaller.
Nephiline
radius con-
struction
behavior
(F2),
with
its
strong
emphasis on radii
originating on
the tem-
porary spiral
rather
than at
the hub
may
also
be
adapted
to
produce
tightly meshed
webs
(viz.
similar
radius
patterns
in
the
very
tightly
meshed
webs of
Cyrtophora
and
Mecynogea).
At
least
until
more
ne-
philine
webs
are
discovered
and
de-
scribed, one
can
tentatively
suppose
that
some of
the
unique behavioral
characters
of
this
group
represent
adaptations
related
to
spinning
tightly
meshed orbs.
This
interpretation
is
contrary
to
the
schemes of web
evolution
proposed
by
Kaston
(1964)
and
Kullmann
(1972)
be-
cause it
has the
distinctive
nephiline webs
secondarily
derived from
more
typical
orbs
(autapomorphic)
rather than
ancestral to
the first
typical orbs. The
interpretations
of
Kaston and
Kullmann
were
based on
far
fewer
data than
those
available now.
In
addition
both
authors
took
as
primitive
those
araneid webs
that
most
closely
re-
semble the
webs of certain
species
of
the-
ridiids
and
linyphiids.
There is a
great
variety
of web
forms
however
in
these
families,
especially
in
Theridiidae
(see
for
example
Bristowe,
1958;
also
Marples,
1955a, 1955b;
Kullmann,
1970;
Mascord,
1970;
Forster
and
Forster,
1973;
Eber-
hard,
1977b,
1979;
Carico,
1978;
Clyne,
1979;
pers.
observ. of
Chrysso
spp.
and
Chrosiothes
sp.).
These authors
give no
reason
for
assuming
links
to the
particular
species
they
chose
(other
than that
the
species are common
temperate
forms and
thus better
known).
Their
decision to con-
sider
certain
araneid web
characters
prim-
itive
thus seems
arbitrary.
In
addition the
web character on which
both
place
emphasis-the
presence
of a
mesh
or "barrier"
web on one or
both
sides

SPIDER
BEHAVIOR AND
TAXONOMY
1073
of
the orb-would
seem to have
a
number
of
possible
functions (e.g., Hingston,
1922c; Robinson
and Robinson, 1973; Lu-
bin, 1975) and to
be
a
good example
of
the
non-conservative
characters mentioned by
Levi (1978a). The
presence of barrier webs
near the orbs of
such diverse genera as
Metepeira
(Araneinae, Araneae-e.g., Si-
mon, 1892), Spilasma
(Araneinae, Ara-
neae-pers.
observ.), Arachnura (Ara-
neinae,
Arachnureae-Main, 1976),
Argiope
(Araneinae, Argiopeae-e.g., Lu-
bin, 1975), Gasteracantha
(Araneinae,
Gasteracantheae-pers. observ.),
Leu-
cauge
(Metinae-e.g., Comstock, 1948),
and the uloborid genera
Philoponella (Pe-
ters, 1953;
Eberhard, 1969;
Lahmann
and
Eberhard, 1979;
Opell, 1979) and Ulobo-
rus (Lubin et al.,
unpubl.) as well as in
the nephiline genera
Nephila (Nephileae)
and Phonognatha
(Phonognatheae-
Main, 1976)
indicates
that the
presence
of
a
mesh
cannot be
taken
as a reliable
in-
dication
that
other
aspects
of that
species'
web
are
primitive.
A
further difference
between the scheme
proposed here
and
those
of Kaston and
Kullmann is my
placement of Cyrtophora
and
Mecynogea
(=Allepeira)
as
a
deriva-
tive group within
the Araneinae, far
from
the ancestral stem
of
the orb weavers. The
objections to
their
choice
of
primitive
character states
apply
here also.
In
addi-
tion,
while the
building
behavior data are
limited (these genera do not
spin sticky
spirals
and have
distinctive
radius
con-
struction
behavior),
the existence
of both
F1
and F3 radius construction behavior
in
Argiope
anasuja,
a
member
of
a
genus
closely related
to
Cyrtophora
in
its adult
morphology (Levi,
1978),
attack behavior
(Robinson, 1975),
and
egg
sac form
(Si-
mon, 1892)
is
in
accord with
a
derived
position
for
Cyrtophora
and
Mecynogea.
Robinson and Robinson
(1978) suggested
placing Argiope near the base
of
the ara-
neid line on the
basis of mating behavior
(mating site).
Their
argument
is not con-
vincing
since this character seems to be
non-conservative
(see Appendix 3)
and
since,
as
they
themselves
point out,
it
is
not
possible
to establish whether the more
simple type
of
courtship
is
primitive
or
derived. They imply that Argiope may be
close to Nephilinae, but it is clear that the
data
here do not support such a grouping.
Attack behavior (Robinson, 1975; Appen-
dix
3)
and
the occurrence of tarsal rubbing
in male courtship as well as other details
of
mating
behavior
(Robinson and Rob-
inson, 1980) also argue for separation of
the two groups and a relatively more de-
rived position for Argiope.
Some
authors (e.g., Kaston, 1948; For-
ster, 1967; Lehtinen, 1967, 1974)
have
placed tetragnathines in a separate family,
but
the data
here could only support
this
classification
if
metines
were
also placed
in
this
separate family and
if
the hitherto
unquestioned
araneid
group Nephilinae
were
put
in
its
own
family. Lehtinen (1975)
proposed, on the basis of the structure
of
genital organs,
trichobothrial
patterns
on
legs, and color patterns (no precise data
were given however), that Metinae and
Leucauginae be split from Tetragnathidae
and combined with
Nesticidae and
Mi-
metidae
in
a family separate from both
Araneidae and Tetragnathidae. The data
here
do
not
support
this
split since
Leu-
cauge
and
other metines seem identical
to
the
tetragnathines
in
all the
characters
ex-
amined.
In
addition
they
share the con-
struction
of
orb webs with
Tetragnathinae
but
not
Nesticidae
and
Mimetidae.
One
would have
to
postulate
a
secondary
loss
of orb webs
in
nesticids and
mimetids
to
justify grouping
them with
the
orb weav-
ers.
SUMMARY
Some
details of orb web
construction
and attack behavior
are
evolutionarily
conservative
and
appear
to be useful
in
defining
subfamilies and families and
de-
termining relationships.
Their
patterns
of
distribution
among
the
at least 148
species
in
at least 55
genera surveyed
here
agree
in
general
with
classical taxonomic
schemes based
on
adult
morphology.
The
data
suggest
that
convergent
evolution
of
orb
webs
may
have
occurred
in
two lines
(uloborids
and
araneoids). They
also
in-
dicate
that several
previous proposals
re-

1074
WILLIAM
G.
EBERHARD
garding the evolution of orb weavers and
their webs may be incorrect. Certain be-
haviors appear to constitute autapomor-
phies for Uloboridae, Nephilinae, and
Araneinae, while others may be synapo-
morphies for Theridiosomatidae-Anapi-
dae.
ACKNOWLEDGMENTS
Dr.
Herbert W. Levi spent many hours
identifying long series of araneid spiders
for
me. Without his dedicated and invalu-
able aid this
project would
never have
been begun.
I
hope that the results pre-
sented here help make his
effort
seem
worthwhile.
I
am also grateful to Drs. Norman Plat-
nick and Brent Opell for identifications of
anapids
and
uloborids respectively,
to
Jonathan Coddington
for tentative names
for
theridiosomatids,
and
Dr. P. N.
Witt
for
sending specimens
of Mallos
gregalis.
I
benefitted greatly from timely discus-
sions with
Dr.
Platnick and
J. Codding-
ton. They, along
with H.
W. Levi,
C.
E.
Valerio,
B. P.
Opell
and
M.
J. West
Eber-
hard read preliminary versions of the
manuscript.
The observations were made over a
span
of about
10
years, during
which time
I
received aid from many people.
I
am
especially grateful
to Central
Hidroelec-
trica
Anchicaya', Carlos Rodriguez,
the
Dixon Stroud family, and Drs. Luis
Ar-
ango,
Carlos
Valerio,
Madhav
Gadgil,
and
A.
J.
T.
Johnsingh for help
and hos-
pitality.
The work was financed by the Comite
de
Investigaciones of
the Universidad del
Valle, Cali, Colombia,
and the Vicerec-
toria de
Investigacion
of the Universidad
de Costa
Rica.
Note
added
in
proof:
Fukumoto
(1973,
1981), working
with
Nephila, Tetragnatha,
and
five
species
in
four
araneinine
genera
(probably
all
species
were different
from
those
in
this
study) recognized
some
of the
similarities and differences
in
sticky spiral
construction
(characters A, B,
and
C)
which are documented
here.
I
thank
M.
Stowe for
kindly translating
these articles
from
Japanese.
LITERATURE
CITED
BRIGNOLI,
P.
M. 1979.
Contribution
i
la
connais-
sance
des
Uloboridae
palearctiques
(Araneae).
Rev.
Arachnol.
2(6):275-282.
BRISTOWE,
W.
S. 1958.
The
World
of
Spiders.
Col-
lins,
London.
304
p.
CARICO,
J. E. 1978. Predatory
behavior
in
Euryopis
funebris
(Hentz)
(Araneae:
Theridiidae)
and
the
evolutionary
significance
of
web
reduction,
p.
51-58.
In
P.
Merrett
(ed.),
Arachnology,
Symp.
Zool.
Soc.
London
42.
CLYNE,
D.
1973.
Notes
on the
web
of
Poecilopachys
australasia
(Griffith
and
Pidgeon,
1833)
(Aranei-
da:
Argiopidae).
Aust.
Ent.
Mag. 1(3):23-29.
.
1975.
Come
into
my parlour,
said
the spi-
der;
techniques
of spider
predation.
Mantis
Wild-
life
Films,
Turramurra
2074,
N.
S.
W.,
Australia.
1979.
The
Garden
Jungle.
Collins,
London.
184 p.
COMSTOCK,
J.
H. 1940.
The Spider
Book.
Revised
and
edited by
W.
J.
Gertsch.
Comstock
Pub.
Assoc.,
Ithaca.
729 p.
CRANE,
J.
1975.
Fiddler
Crabs
of
the
World
(Ocy-
podidae:
Genus
Uca).
Princeton
Univ.
Press,
Princeton.
736 p.
CROME,
W.
1954.
Beschreibung,
Morphologie
und
Lebensweise
der
Eucta
kaestneri sp.
n.
(Araneae,
Tetragnathidae).
Zool.
Jb.
(Syst.)
82:425-452.
DARWIN,
C.
1859.
On
the
Origin
of
Species
by
Means
of
Natural
Selection,
1st ed. John
Murray,
London.
503
p.
DENNY,
M.
1976.
The
physical
properties
of
spider's
silk
and
their
role
in the
design
of orb webs.
J.
Exp.
Biol.
65:483-506.
EBERHARD,
W.
G.
1967.
Attack
behavior
of
digue-
tid
spiders
and
the
origin
of
prey
wrapping
in
spiders.
Psyche
74:173-181.
.
1969.
The
spider
Uloborus
diversus Marx
and its
web.
Ph.D. thesis.
Harvard
Univ.,
Cam-
bridge.
.
1972.
The
web
of
Uloborus
diversus
(Ara-
neae:
Uloboridae).
J.
Zool.,
London
166:417-
465.
.
1976.
Physical
properties
of sticky
spirals
and
their
connections: sliding
connections
in
orb
webs.
J.
Nat.
Hist.
10:481-488.
.
1977a.
Aggressive
chemical
mimicry
by
a
bolas
spider.
Science
198:1173-1175.
.
197
7b.
'Rectangular
orb'
webs
of
Synotaxus
(Araneae:
Theridiidae).
J.
Nat.
Hist.
11:501-507.
.
1979.
Argyrodes
attenuatus (Theridiidae):
a web
that
is
not a snare.
Psyche
86:407-413.
.
198 1a.
The
single
line web
of Phoron-
cidia
studo
Levi: a
prey
attractant
(Araneae,
Theridiidae)? J.
Arachnol.
9:229-232.
1981b.
Notes
on
the natural history
of
Taczanowskia
sp.
(Araneae:
Araneidae).
Bull.
Br.
Arachnol.
Soc.
5(4):175-176.
.
1981c.
Building
behavior
and web
ten-
sions
in orb
weavers.
Bull.
Br. Arachnol.
Soc.
5(5):
189-204.
EICKWORT,
G.
C.,
AND
S.
F.
SAKAGAMI.
1979.
A
classification
of nest
architecture
of
bees
in
the
tribe
Augochlorini
(Hymenoptera:
Halictidae;

SPIDER
BEHAVIOR
AND
TAXONOMY
1075
Halictinae), with
description of a
Brazilian nest
of Rhinocorynura
inflaticeps.
Biotropica
11:28-
37.
EVANS, H. E.
1966. Comparative Ethology
and
Evolution of the
Sand Wasps. Harvard
Univ.
Press, Cambridge.
526 p.
FORSTER, R. R.
1967. The Spiders of
New Zealand.
Part I. Otago
Mus. Bull. 1:7-124.
.
1970.
The Spiders of New Zealand.
Part
III. Otago Mus. Bull. 3:11-184.
FORSTER, R. R.,
AND L. M. FORSTER.
1973. New
Zealand Spiders.
Collins, London.
254 p.
FORSTER,
R.
R.,
AND
N. I.
PLATNICK. 1977. A
re-
view of
the
spider
family Symphytognathidae
(Arachnida,
Araneae). Amer. Mus.
Novit.
2619:1-
29.
GORNER, P.
1966. Uber die Koppelung
der
optisch-
en
und kinasthetischen
Orientierung
bei
den
Trichterspinnen
Agelena labyrinthica (Clerck)
und
Agelena gracilens
C.
L.
Koch.
Z.
vergl. Physiol.
53:253-276.
GREENE, A. 1979.
Behavioral characters
as indi-
cators of yellow
jacket phylogeny (Hymenoptera:
Vespidae).
Ann.
Entomol.
Soc.
Amer.
72:614-
619.
HINGSTON,
R. W.
G. 1922a. The snare
of the giant
wood
spider (Nephila
maculata).
Part I.
J.
Bom-
bay Nat. Hist.
Soc. 28(3):642-649.
. 192
2b.
The snare of the giant
wood spider
(Nephila
maculata).
Part II.
J.
Bombay Nat.
Hist. Soc. 28(4):911-917.
.
1922c. The snare
of
the giant
wood
spider
(Nephila
maculata).
Part III.
J.
Bombay Nat.
Hist. Soc. 28(4):917-923.
HOLM, A.
1939.
Beitriige zur Biologie
der Theridi-
iden. Festr. Strand
5:56-67.
JACOBI-KLEEMANN,
M. 1953. Ueber die
Locomo-
tion
der Kreuzspinne
Aranea
diademata
beim
Netzbau (nach
Filmanalysen).
Z.
vergl. Physiol.
34:606-654.
KASTON, B. J. 1948.
Spiders of Connecticut.
Conn.
St.
Geol.
Nat. Hist.
Survey
Bull.
70:5-874.
.
1964.
The evolution of spider
webs. Amer.
Zool. 4:191-207.
KULLMANN,
E. 1971. Bemerkenswerte
Konvergen-
zen im Verhalten
cribellater und ecribellater
Spinnen.
Freunde
Kolner
Zoo
13(4):123-150.
. 1972.
The
convergent development
of orb-
webs
in cribellate and ecribellate
spiders.
Amer.
Zool. 12:395-405.
KULLMANN, E.,
AND
W.
ZIMMERMANN.
1976.
Ein
neuer
Beitrag zum Cribellaten-Ecribellaten
Problem: Beischreibung
von
Uroecobius
ecribel-
latus n. gen.
n. sp. und Diskussion
seiner phy-
logenetischen
Stellung.
Ent. Germ.
3(1/2):29-40.
LAHMANN, E.,
AND
W. EBERHARD.
1979.
Factores
selectivos
que
afectan
la tendencia
a
agruparse
en la
arafia
colonial
Philoponella
semiplumosa
(Araneae;
Uloboridae).
Rev.
Biol.
Trop.
2
7(2):23
1-240.
LEHTINEN,
P.
1967.
Classification
of the cribellate
spiders
and
some allied
families,
with notes on
the
evolution
of the suborder
Araneomorpha.
Ann.
Zool.
Fenn. 4:199-468.
.
1975. Notes on the
phylogenetic classifica-
tion
of
Araneae.
Proc. 7th Int.
Arachnol.
Congr.
:26-2
9.
LEVI, H. W. 1968. The
spider genera Gea and Ar-
giope
in
America
(Araneae: Araneidae). Bull.
Mus. Comp. Zool.
136:319-352.
.
1978a. Orb-webs
and phylogeny of orb-
weavers, p.
1-15.
In
P.
Merrett (ed.), Arachnol-
ogy, Symp. Zool.
Soc.
London 42.
.
1978b. Orb-weaving
spiders and their webs.
Amer. Sci. 66:734-742.
.
1980a. The
orb-weaver genus Mecynogea,
the subfamily Metinae, and
the genera Pachy-
gnatha, Glenognatha,
and Azilia of the subfamily
Tetragnathinae north
of Mexico. Bull. Mus.
Comp. Zool.
149:1-75.
.
1980b. Orb-webs:
primitive or specialized.
Proc' 8th Int. Arachnol.
Congr. (Vienna):367-
370.
LONGMAN, H. 1922. The
magnificent spider:
Di-
chrostichus magnificus
Rainbow.
Proc.
Roy. Soc.
Queensland
33:91-98.
LUBIN,
Y.
D.
1973.
Web
structure and function: the
non-adhesive
orb-web of
Cyrtophora
muluccensis
(Doleschall)
(Araneae:Araneidae). Forma
et
Functio 6:337-358.
.
1975. Stabilimenta
and barrier webs in the
orb
webs
of
Argiope argentata
(Araneae,
Aranei-
dae)
on
Daphne
and
Santa Cruz
Islands,
Gala-
pagos. J.
Arachnol.
2:119-126.
LUBIN,
Y.
D.,
W. G.
EBERHARD,
AND
G. G.
MONT-
GOMERY.
1978. Webs of
Miagrammopes (Ara-
neae: Uloboridae)
in
the
neotropics. Psyche
85:1-
23.
MAIN,
B.
1976. Spiders.
Collins, London. 296 p.
MARPLES, B. J. 1955a.
A
new type of web spun by
spiders
of the
genus
Ulesanis
with the
description
of
two
new
species.
Proc. Zool. Soc. London
125:75
1-760.
1955b. Spiders
from Western Samoa. J.
Linn. Soc.
Zool. 42:453-504.
.
1962. Notes on
spiders of the family Ulo-
boridae.
Ann. Zool.
(Agra.) 4(1):1-11.
MARPLES, M. J.,
AND
B. J. MARPLES. 1937. Notes
on
the
spiders Hyptiotes
paradoxus
and
Cyclosa
conica. Proc. Zool. Soc.
London Series
A
1937:2
13-22 1.
McKEOWN, K. C. 1952.
Australian Spiders. Angus
&
Robertson,
London.
287
p.
MASCORD,
R.
1970. Australian
Spiders.
Ch. E.
Tut-
tle
& Co., Rutland,
Vt.
MICHENER,
C.
D.,
M.
L.
WINSTON,
AND
R. JAN-
DER. 1978. Pollen
manipulation and related ac-
tivities
and
structures
in
bees of
the
family Api-
dae.
Univ. Kansas
Sci.
Bull.
51:575-601.
MUMA,
M.
1971.
Biological
and
behavioral notes
on
Gasteracantha
cancriformis
(Arachnida:
Aranei-
dae).
Fla.
Entomol.
54:345-351.
OPELL, B. P. 1979. Revision of
the genera and trop-
ical
American
species
of the
spider family
Ulo-
boridae.
Bull. Mus.
Comp. Zool. 148:443-549.
PEAKALL,
D.
B.,
AND
P.
N. WITT.
1976.
The en-
ergy budget
of an
orb
web-building spider. Comp.
Biochem. Physiol.
54A:187-190.

1076
WILLIAM
G.
EBERHARD
PETERS,
H. 1931.
Die Fanghandlung
der
Kreuz-
spinne
(Epeira
diademata
L.).
Experimentelle
Analysen
des
Verhaltens.
Z.
vergl.
Physiol.
15 :693-748.
.
1953.
Beitriige
zur
vergleichenden
Etholo-
gie
und Oekologie
tropischen
Webespinnen.
Z.
Morph.
Okol.
Tiere
42:278-306.
.
1954.
Estudios
adicionales
sobre
la
estruc-
tura
de la
red concentrica
de
las arafias.
Comun-
ic. Inst. trop.
Invest.
cien.
Univ.
Salvador 3(1):
1-
18.
PETRUNKEVITCH,
A. 1926.
The value
of instinct
as
a taxonomic
character
in
spiders.
Biol.
Bull.
50:427-432.
RISCH,
P. 1977.
Quantitative
analyses
of
orb-web
patterns
in four species
of spiders.
Behav.
Genet.
7:
199-238.
ROBINSON,
M.
H.
1969.
Predatory
behavior
of
Ar-
giope
argentata
(Fabricius).
Amer. Zool.
9:161-
173.
.
1975.
The
evolution
of
predatory
behavior
in
araneid
spiders,
p.
292-312.
In
G. Baerends,
C.
Beer,
and
A. Manning
(eds.),
Function
and
Evolution
in
Behaviour.
Clarendon
Press,
Ox-
ford.
ROBINSON,
M.
H.,
AND Y.
D.
LUBIN. 1979a. Spe-
cialists and
generalists:
the
ecology
and behavior
of some
web-building
spiders
from
Papua
New
Guinea.
I. Herennia
ornatissima,
Argiope
ocy-
aloides,
and
Arachnura
melanura (Araneae:
Ar-
aneidae).
Pac. Ins. 21:97-132.
.
1979b.
Specialists
and
generalists:
the ecol-
ogy
and
behavior of
some web-building spiders
from
Papua
New
Guinea.
II.
Psechrus
argenta-
tus
and Fecenia
sp. (Araneae:
Psechridae).
Pac.
Ins.
21:133-164.
ROBINSON,
M. H.,
AND
J. OLAZARRI.
1971.
Units
of behavior
and
complex
sequences
in the
pred-
atory
behavior
of
Argiope
argentata
(Fabricius):
(Araneae:
Araneidae).
Smithsonian
Contr.
Zool.
65:1-36.
ROBINSON,
M.
H.,
AND
B. ROBINSON.
1973. Ecol-
ogy
and
behavior of the
giant
wood
spider
Ne-
phila
maculata
(Fabricius)
in New Guinea.
Smithsonian
Contr.
Zool. 149:1-76.
.
1975.
Evolution
beyond
the orb web:
the
web of the
araneid
spider
Pasilobus
sp.,
its struc-
ture, operation
and construction.
Zool.
J.
Linn.
Soc.
56(4):301-314.
.
1978.
The evolution
of courtship
systems
in
tropical
araneid
spiders,
p.
17-29.
In
P. Merrett
(ed.),
Arachnology,
Symp.
Zool.
Soc. London
42.
.
1980. Comparative
studies
of the courtship
and
mating
behavior of
tropical
araneid
spiders.
Pac. Ins.
Monogr.
36:1-218.
ROBINSON,
M. H., B. ROBINSON,
AND
W. GRANEY.
1974. The
predatory
behavior
of
the nocturnal
orb web
spider
Eriophorafuliginea
(C.
L.
Koch)
(Araneae:
Araneidae).
Rev.
Per. Entomol.
14(2):304-3
15.
ROVNER, J.,
AND
S.
J.
KNOST. 1974. Post-immo-
bilization
wrapping
of
prey
by lycosid
spiders
of
the herbaceous
stratum.
Psyche
81:398-415.
SEYFARTH,
E.,
AND
F.
BARTH.
1972.
Compound
slit sense organs
on the
spider
leg:
mechanore-
ceptors involved
in
kinesthetic
orientation.
J.
Comp. Physiol. 78:176-191.
SIMON,
E. 1892. Histoire Naturelle des
Araignees.
I.
2nd ed. Lib. Encycloped. Roret,
Paris. 1084 p.
STOWE, M. 1978. Observations of two
nocturnal orb
weavers
that
build
specialized
webs:
Scoloderus
cordatus and Wixia ectypa (Araneae:
Araneidae).
J. Arachnol. 6:141-146.
WIEHLE, H. 1928.
Beitriige
zur Biologie
der Ara-
neen, inbesondere zur
Kenntnis des Radnetz-
baues. Z. Morph.
Okol. Tiere 15:262-306.
.
1931. Neue Beitriige zur
Kenntnis des
Fanggewebes der Spinnen aus den
Familien
Ar-
giopidae, Uloboridae, und Theridiidae.
Z.
Morph.
Okol. Tiere
22:348-400.
WITT, P. N. 1965. Do we live in
the best of all
possible
worlds?
Spider
webs
suggest
an answer.
Perspect. Biol. Med. 8:475-487.
WITT, P. N., C. REED,
AND
D. B.
PEAKALL.
1968.
A
Spider's Web. Springer,
N.Y.
107
p.
Corresponding
Editor:
D.
J.
Futuyma
APPENDIX 1.
Behavior
descriptions
and probable
directions of change
Spiders
were observed
in the wild and in a
large
screen
cage.
Those building
in
the
dark were illu-
minated at least
periodically
with a headlamp.
Super
8
movies were
made of several
species
as
they
spun
sticky spiral
(see Appendix
2) and
were analyzed
frame by frame.
The positions
of web threads
were
sufficiently
clear
in
the movies to
give good
confi-
dence that the
drawings made
from them (Figs.
2-4)
are
precise,
and enough repetitions
observed
to
as-
certain that
the behavior described
is
typical
and
highly stereotyped.
In
no
case
did
I
see
significant
variation among
individuals of a single species
in the
behavior patterns
described
here except
for the
dif-
ferences
(noted
in
Appendix
2)
between
young
and
old
individuals
of
some
Nephilinae
and two
species
of
Micrathena in character
A
and some
araneines
in
character
D. Most observations
were
of mature fe-
males.
The characters
are denoted
by letters,
and the
character states
by
numbers (e.g.,
character
B
has
two states,
B1
and B2).
The descriptions
are
orga-
nized with respect
to the stage
of web construction
in
which the
behavior occurs.
The probable
func-
tions of some
of the behaviors are
discussed else-
where
(Eberhard,
1981c).
I. Sticky Spiral
Construction
All orb weavers
whose behavior is known
place
the
sticky
thread
on
their webs after
building
a "scaf-
fold" of
radii,
frame
lines,
hub,
and
(with
some ex-
ceptions)
temporary
non-sticky spiral
lines.
They
start the sticky spiral
near
the
edge
of
the web and
gradually
work
inward.
Figure
1
illustrates
this
pro-
cess,
and
gives
the
names
used
for the various lines

SPIDER
BEHAVIOR AND
TAXONOMY
1077
and
legs
used
in
the behavioral
descriptions
which
follow.
A. Determination
of sticky spiral attachment
point
A1:
Tap forward
with
leg
iI
(Fig. 2).
The
spider
faced inward as she attached to
rN, proceeded
toward
the
hub,
and
then
turned
to
face out the
next
radius
(rN+1)
and moved
forward rather than
sideways out
to
encounter the
inner loop, finally turning
1800
to
attach
again. This
abrupt turning to face
inward
and
then outward was
especially
dramatic as the
spider
laid sticky spiral
near the hub. As the spider
moved
out
rN+l,
iI
tapped
or
pushed forward to contact the
inner
loop while ol was held in
nearly
the same
po-
sition but
was involved
in
gripping
the radius.
A2: Tap
sideways with leg oI (Fig. 3).
The spider
moved sideways
away
from
the hub along
rN+l
wav-
ing
or
tapping
repeatedly
with
the outer of her front
legs which was directed
laterally to
the side toward
which she
was
moving (Fig. 2).
Her
body
was
thus
oriented
perpendicular
to
the
radius,
and her outer
leg
I
was more or
less parallel to it.
A3: Extend leg
oIV backward (Fig. 4). Instead of
moving inward
toward the hub and then
back out-
ward,
the
spider
moved
almost
directly
from
one
attachment to the
next, sidling
across the web as she
faced inward toward the hub. The front
legs
were
not brought near the site
where an attachment
was
about
to
be
made, although
oII sometimes
tapped
toward the inner
loop
several radii
in
advance
of
the
current
attachment
site. The
spider used
oIV
to
probe
for
the point
where
rN+1
intersected
the
inner
loop.
A4: No contact.
The spider's body was small com-
pared
to
the
spaces
between radii and between
loops
of
sticky spiral.
After
attaching
to
rN
and
moving
inward
to
reach
rN+l,
the
spider
faced outward
as
she moved
away
from the hub
along
rN+l,
but
stopped
when still
several
body lengths
short
of the inner
loop
and
turned
to attach.
B.
Legs holding
rN+1
near
attachment
point
when
attachment made
B
1:
oIII
just
inside the attachment
point,
oIV
just
outside
it.
As the
spider
neared
the attachment
point,
leg
oIII
seized
rN+1
just
inside
the
point
where
the
attachment was to
be made.
Then as the
spider
po-
sitioned
her
abdomen to make the
attachment,
oIV
grabbed the radius
just
outside the attachment
point
(Figs. 2, 3, 4).
B2: oIV just
outside
the
attachment point
and
iIV
just
inside
it. The
spider
ceased
combing
sticky
cri-
bellum
silk
with her
legs
IV
just
before
making
the
attachment,
and
gripped rN+1
on either side
of
the
attachment
point
with these
legs
as she
faced
inward.
C. Push
current
segment
with iIV
just
before
at-
taching
C1: Push the
line. Leg
iIV
was extended
(Fig. 4)
and/or bent
ventrally (Fig. 2) just
before the attach-
ment was
made;
in
each case the
tip
of the
leg
moved
away
from
the
spinnerets,
so
additional
line
was
probably
drawn out
(see Eberhard,
1981
c).
In
TEMPORARY SPIRAL
-
- jV
\
INNER
LOOP
CURRENT SEGMENT
F,,
RN
RN+1
FIG. 1.
Path
taken by a
typical
araneid as it
moves
between one
attachment
of the
sticky spiral
and the next
(sticky
spiral lines
shown
thicker
than
others).
The
line
is
attached to
radius rN at
point x,
and
then the
spider
moves (dotted
line) to
radius
rN+1
via the
outermost
loop
of
temporary spiral. It then
moves
out
rN+j
and
attaches the
current
segment of
sticky spiral
(the
line which it
has produced as it
moves) at
point y.
The size of
the spider
compared
to the
distances
between radii and
between the
sticky
spiral
loops
is
relatively
constant
within
species but
varies
widely between them. The
legs designated as
inner
(i)
are
closer to the
hub,
and
the outer
(o)
ones
closer to the
web's
edge.
When
the spider doubles
back
(i.e.,
moves
clockwise
instead of
counterclock-
wise
in
the
figure),
i
legs
become
o
legs and vice
versa.
Nephila
clavipes,
Micrathena
gracilis and
M. sex-
spinosa
I
was
able to
ascertain
that the
exact point
where the
sticky
line
contacted
the
leg
was
variable,
and
was
usually
on
the side rather
than the
tip
of the
tarsus.
It
appeared
that
the line
snagged
on
the stiff
hairs
covering
the
sides
of the
leg.
C2:
Sticky line not
pushed with
legs. The
current
segment
was
not touched
by
any leg
as it
was at-
tached to
rN+l.
D.
Contact with
temporary spiral
as lay
sticky spiral
Dl:
Lose
contact. The
spider moved
beyond the
outermost
loop
of
temporary spiral
and was com-
pletely out
of
contact with it as she
attached
at least
some
of
the
outermost
loops
of
sticky spiral.
D2: Maintain
contact. The spider
maintained con-
tact with the
temporary spiral
at all
times while
spin-
ning
sticky spiral.
The
outline
of
the area covered
by
sticky
silk
thus more or less
reflected the
outline
of
the
outer
loop
of
temporary spiral.
D3: No
temporary
spiral. There
was no
temporary
spiral
in
the web.

1078
WILLIAM
G. EBERHARD
3
6
2
20
tsp
olV
t
4X
inner
oi
Rn
Rn.1l
Rn
Rn+o
16
17
20
21
tsp
tst
oil
ii
'~~~current
o
ol
segment
o lR
inner
lo
oil
l
oop-
i
Rn
Rnw+s Rn
Rn+1
22
24
25
tp
30
tsp
tsp
II ~~~~~~~~~~IIV
olil
IV
ollI-
~~~~~~~~~~~~~current
inner
oil
current)
oIl
sd6ment-en
loop-,0
o
emn
oIl oV
0lV
I
'Rn
Rni1
Rn
Rn+1
FIG.
2.
Al
determination
of
sticky
spiral
attachment
point
behavior,
illustrated from
films of
Leucauge
sp.
(#1556) taken
looking
down
at the
spider
as
it
moved
counterclockwise
on a horizontal web.
Conventions
are
as described for
Figure
3.
Body:
faced inward
as
attachment
was
made
to
RN (2)
and as
the
spider moved
inward
along
RN
(3,
6);
then turned
to
face
more
or less
along
the
temporary
spiral
(11),
and
then
away
from
the hub
as the
spider
moved out
RN+1
(16-2
1);
finally
it
turned
1800
briskly (2 2) just
after
leg
ii
touched
the
inner
loop
and
stayed
in
this
position
as
the
attachment was
made.
Leg
pi:
held
RN
as the
attachment
was
made;
after
variable behavior while the
spider
moved in
and across the
temporary
spiral,
it
was
extended
forward and
was bent
ventrally
at the mt-tibia
joint
as 'the
spider
moved
out
RN+1
(16,
17)
until
it
made
contact
with
the
inner
loop (20),
then
swung
back
to
seize
RN+1
(24)
as the
spider
turned
to
make
the
attachment.
Legs
ol and
oil: held
nothing
while the
attachment
was
made
(2);
waved
and
contacted
RN+1
as the
spider
moved
inward toward
the
hub and
across
the
temporar-y spiral bridge,
then
moved
along
RN+s
in
a
"hand-over-hand"
pattern (e.g.,
17, 20);
as soon
as
leg
il
touched
the
inner
loop, leg
oI
swung
to
the
side
as the
spider began
its 1800 turn
(21,
22),
but
leg
oil
retained its hold on
RN+1
until
oII
and
oIV
had
seized
this
thread
(24),
and then it
also
swung
to the
side
(25).
Leg
oIV:
held
RN just
beyond
the
attachment
point
as the
attachment was
made
(2);
retalned its hold
on
RN
as
the
spider
moved inward
(3,
6),
then seized
the
current
loop
of
sticky
spiral (16),
pulled
it
once
(17),
then held
it
as the
spider
moved
out
RN?l,
finally
releasing
its hold
(22)
and
moving
to seize
RN+S
just
outside the
point
where
oil
was
holding
it
(24);
it
maintalned
this
hold until the
attachment
was made.
Leg
iIV: after
releasing
the
sticky spiral
line
(generally
"plucking"
it with
a
snap--3),
moved
irregularly
until
oIV released
its hold on the
current
segment (24)
and,
just
before the
attachment was
made,
pulled
out
more
silk
by
extending
with a
straightening
at
the
femur-
patella-tibia
joints
(30).

SPIDER
BEHAVIOR
AND
TAXONOMY
1079
E. Attach sticky
spiral
to each radius
crossed
El: Attach
to each one.
The
spider
attached the
sticky line to
each radius
she crossed
as she moved
around the
web. Apparent
exceptions
occurred
only
on webs
in
which all radii
were not in the same
plane
and the spider
did not
"encounter" all radii
which
crossed her
path.
E2: Skip
some radii. The
spider crossed some
radii
as she laid sticky spiral
without
attaching
to them.
This behavior,
which can be
deduced from
finished
webs, was
especially common
near the
hub.
II. Radius
Construction
F.
Order of thread placement
F1:
One trip from hub-one
radius laid (Fig. 5).
Starting
from the hub,
the
spider
moved to the
edge
of the web along a pre-existing
radius, paying
out
a
new line as
she went. She
attached this
line to the
frame, but
then immediately
broke
it
and
rolled it
up
as
she returned to the
hub, laying
a
replacement
line behind
her as she
went.
Usually
radius
con-
struction was uninterrupted
by
hub
construction,
and
the hub
loops
were
laid
only
late
in
the radius
construction stage
or after
it
was finished.
F2:
One
trip from hub-two
radii attached
at two
points
on the frame
(Fig.
6). Starting
either at the
hub as she
laid hub loops
or at some point
away
from the
hub as she spun
the temporary spiral,
the
spider
moved to the
edge
of the
web, paying
out
a
new line as
she went.
She attached this
line to the
frame, then
moved farther
along the frame
and at-
tached it
again;
finally
she used
the line laid on the
way
out for
support
as
she returned to the
hub, lay-
ing a second
new radius
as she went. On arriving
at
the point of
origin she
resumed laying hub
or tem-
porary spiral
line. Thus
in
contrast to the
preceding
case,
hub
loop
construction
was an
integral
part
of
radius construction.
F3:
One
trip
from the
hub-two radii attached at
a
single point
on the frame
(Fig.
7). Cyrtophora
webs
have no
sticky spiral,
but
they
do have
very tightly
spaced
radii. These were
laid two at a time
along
with hub
and
"temporary
spiral" lines,
but differed
from
F2
behavior
in
being
attached
to the frame only
once instead of
twice,
and
to the hub or
temporary
spiral
at
two
points
rather
than one. This
type
of
behavior could be deduced
from
inspection
of fin-
ished webs since
it
results
in
characteristic "V" shaped
intersections of radii with
frame threads.
F4:
One
trip
from
the hub-one double radius
(Fig. 8).
Interrupting
hub construction,
the
spider
walked from the hub along
a
pre-existing
radius and
attached the line she laid
behind her to the
frame,
then returned to the
hub
along
this
line, laying
another behind which was
attached to this one when
the
spider
resumed hub
construction and
which ef-
fectively
doubled the
new radius.
Ill. Hub
Destruction and
Replacement
Gi: Hub left intact.
The
spider
left the hub
of the
web intact after
finishing
the
sticky spiral.
G2:
Hub center removed. The
spider
moved to the
hub
and apparently
ingested
the
threads
in
the
very
center,
and
left this hole
in
the
finished web.
G3:
Hub center removed
and
replaced.
After
mak-
ing a
hole at the center of the hub the
spider
laid
additional
lines which more or less
"sewed
up"
the
hole
she
had created.
G4:
Entire hub removed.
The
spider
destroyed
the
entire
hub rather than
just
the inner
part,
and either
reattached
the radii
directly
to
each other or
made
new
hub loops which were attached to the radii
out-
side the
points
where
they
had been
joined previ-
ously.
G5:
No
hub.
There
was
no
localized area where
radial
lines
converged,
and no lines were
broken
af-
ter
completion
of
the
sticky spiral.
IV.
Temporary
Spiral Form
Hi:
Spiral.
As
the name
indicates, the non-adhe-
sive
line
spun just
after radius
construction and
which
joined
the radii
together away
from the hub
approximated a
spiral, at least near
the hub.
H2:
Circle. The
temporary spiral
consisted of a
single
circle, or,
in
the case of
Epeirotypus (?) sp.
(#2170), two circles,
one inside the
other.
H3:
No
temporary
spiral.
No
temporary spiral
was
spun.
V. Attack Behavior
I1:
Attack all prey
by biting.
Spiders attacked prey
by
biting,
and
only
wrapped them,
if
at all, after a
bite had been
administered.
I2:
Attack wrap
with rotation in
the web. Spiders
attacked at least
some
prey by
wrapping before
bit-
ing.
The wrapping
behavior often
included rotating
the
prey
while
it
was still
attached to
the web (usu-
ally
to a
radius)
so that it
spun
"rotisserie-fashion"
(or
"bobbin-fashion"
in
Robinson,
1969) and
the
turning
movement of
the
prey
itself
appeared to pull
wrapping
silk from the
spider's spinnerets.
When
the
prey
was very large the
spider
ran
around and over
it
rather than
spinning
it.
I3:
Attack wrap
without rotation in
the web. Spi-
ders attacked at least some
prey by
pulling silk
from
their
spinnerets with
legs
IV
and
laying it onto the
prey before
administering
a
bite. The
prey was not
spun
rotisserie-fashion while it
was still
attached
to
the
web,
but
was sometimes
rotated
slowly while
being
wrapped
after
it
had been cut
free from the
web.
I4: Attack
wrap with
rotation
unspecified. Most
accounts
of
attack
wrapping
in
the
literature do not
specify
whether
the
prey
was rotated
while
in
the
web or
not.
Species
for which
only
this
type
of
in-
formation
is available
are
assigned
to
this
category.
This
is
thus not a
distinct
character
state, but rather
is
equivalent
to
"either
2
or 3."
It
should
be
noted that attack
behavior
varies
with
the
identity
of the
prey (e.g.,
Robinson, 1969)
and
is
thus
more difficult to
characterize
than web-build-
ing
behavior.
Failure to
observe a
given
type
of at-
tack
may signify
that the
spider
is
incapable
of
that

1080
WILLIAM G. EBERHARD
0
12
20
28
Rn
olV
olV
oXl
ol
l n
;
inner
loop
Rn+
tsp
Rn
32
36
44
52
Current
segment~
l
Rnfps
of oa
tsp oIl
oil
Rn
0
88
92
Rnio
Rn+i
olI li lVI
Rn+1
olli
IlV
inner
oI0V1
loop
tsp
o
ol
tsp
FIG. 3.
Determination of
sticky spiral
attachment
point
behavior of
type
A2,
illustrated
from
films
(18
fps)
of
Alpaida
rhodomelas
(Tacz.) (#1757)
taken
looking
at
the ventral side
of the
spider
as
it
moved
downward
near the
edge
of
a
nearly vertical web.
Numbers indicate
the
number
of
frames since the last
attachment of
the
sticky spiral
(represented by
thicker
lines)
occurred. The
positions
of
some
of the
legs
(especially legs
III)
were often unclear when
they
crossed
the
body,
and
are not drawn in
some figures;
in
some cases notes
taken in the field
were
used to arrive at
the
descriptions
of both
leg
and
thread positions.
When
legs
moved
rapidly they
were blurred
in the
film, and this is
indicated
in
the
figures
by thickening
of
their
outlines (e.g.,
leg
iII
in frame
20).
The movements of different parts
of
the body are
most conveniently
described
separately;
only
movements of the
legs
which seem to move
in
consistent patterns and were
important
in
the extraction and
placement
of the sticky thread
are
described
(other legs
seemed to be mainly
involved
in
supporting the spider
in
the
web).
Body: faced inward as
the
attachment
was made to rN (0),
and
as
the
spider
moved
inward
along rN (12, 20);
then it turned to
face
more or less along the temporary
spiral (28, 32)
and maintained this
position
as
rN+1
was reeled
in
(44-88);
finally
it
turned 900 (just
starting
in
100)
to face inward
again
while the attachment
was made to
RN+,.
Leg oI: was held
in
the air near
RN
while
the
attachment was
made
(0-12),
then
moved variably as the
spider
walked inward along RN and
along
the
temporary spiral (20-28);
just
after oII contacted
RN+,
oI was extended laterally
parallel to
RN+,
(32) and tapped
several times until
it touched the inner
loop of sticky
spiral (88); then it
was brought back
and held
in
front
of
the
spider
and remained relatively
quiet and out of
contact with threads
as the attachment
was made.
Leg
oII: was held near
RN
as the attachment
was
made and as the spider started
inward (12, 20);
after
irregular
movements it contacted
RN+,
and
moved
out this line and
then reeled it in
using a "hand-over-
hand" motion
in
conjunction with
oIII, each leg
releasing the line just
after the other grasped
it, and then
extending
laterally
to
grab
it
again
and
flex
ventrally so
the
leg tip
was brought close to
the spider's body
just
as the other
leg
released its hold and reached
out
laterally
for the
next
grip (various
stages illustrated
in
32-52). Just
after leg
oI
touched
the inner loop (88),
oII
ceased the
hand-over-hand movement
and swung
anteriorly
while the
spider
turned
to make the attachment.
Leg
oIII: held RN just inside
the attachment point
as the attachment was
made
(0),
then moved
irregularly
until
it
began
moving
"hand-over-hand" with oII
along
RN+1;
when
oI
contacted
the
inner loop (88)
it
maintained its grip
on
RN+,
as the spider
turned to make
the
attachment.
Leg
oIV: held RN just beyond the
attachment point
while the attachment
was made (0),
then moved
along RN (12, 20)
before beginning to pull
out sticky spiral
line (e.g., 32, 36) as
the spider moved
toward the next
attachment point.
At
first oIV pulled
silk by itself,
later
in
alternation
with iIV (e.g., 80,

SPIDER
BEHAVIOR
AND
TAXONOMY
1081
kind of behavior,
but
it
may also mean only
that
the
appropriate stimuli
were not present
to
elicit
that
behavior. Only
extensive observations
can distin-
guish
between these
possibilities,
and the data
for
species
of some
groups (particularly
theridiosomatids
and anapids) are as
yet extremely fragmentary.
VI.
Male
Courtship Behavior
Robinson and
Robinson (1980) noted three clear
groupings of
character states associated with mating
site and courtship
mode (direct contact
vs.
thread
vibration).
Unfortunately these patterns seem to have
little to do with
the taxonomic relationships of the
species
involved since
species which are usually placed
in
one small,
distinct group (Argiopeae-the genera
Argiope and
Gea-see Levi, 1968, and also Robinson
and
Robinson,
1980,
for evidence that
these genera
are indeed
closely
related)
fall
in
all
three
of
their
major categories.
One characteristic, however, may
be useful and is
included here. Nearly all the obser-
vations of this character were made
by
Robinson and
Robinson, and
species
are classified
according
to
their criteria.
J. Tarsal Rubbing
by Males
Jl: Without
tarsal rubbing. Males did not perform
tarsal rubbing
(TR) movements while courting fe-
males.
J2: With tarsal
rubbing. Courting males rubbed
their legs together
with
a
motion similar
to that made
when
cleaning
their
legs by rubbing
them
together.
Functional
Independence of
Characters
The characters
B2, C2, E2, and F4 consistently
occurred
only together
(in Uloboridae),
and
it
is
rea-
sonable to ask
if
these are
independent
of
each
other
and of the fact
that all
uloborids
spin
cribellate
sticky
silk.
I
have
argued
elsewhere
(Eberhard, 1976)
that
the
relatively
non-extensible nature of cribellate
sticky
silk
may
indeed be
functionally
related to
E2,
and
believe
it
is
not
unreasonable to
suppose
that
it
is
also related to C2
(see
functional
interpretation
of Cl
in
Eberhard,
1981c).
There is however
no ob-
vious relation between
any
of these
characters
and
B2
and
F4.
The only other
completely consistent combination
was
B1, C1,
and
E1
(in Araneoidea). Again
E1 and
Cl
may
be
functionally
associated with the
very
ex-
tensible
nature
of araneoid orb
weavers'
sticky
silk
and their
ability
to make
"pulley"
connections
to
ra-
dii
(nevertheless, some
groups
do not seem
able to
make such connections-see
Eberhard, 1976).
There
are no
other
obviously necessary relationships.
Character
A3 and
perhaps
also
A4
are
necessarily
associated
with the
relationship
between
web
mesh
size
and
spider body
size:
A3
would
be
impossible
in
relatively widely
meshed webs
(see text) and A4
would
seem less
likely though
not
impossible
in webs
with very small
meshes. These web characteristics
are however
independent
of the other character states.
Probable Directions of Change in
Character States
A. The
probable primitive nature of
Al
with re-
spect
to
A2
is
suggested by
the
exceptional
behavior
of
Tetragnatha sp. (#2043) and Chrysometa species
(#'s 1824, 0-6)
which all
perform
Al
behavior
near
the edges of their webs, but switch to
A2
near the
hubs (Appendix 2). One could consider that
A2 in
these species is either
a
remnant of an ancestral be-
havior Which
has
been lost
in
all
other
observed
Te-
tragnathinae-Metinae (i.e., a symplesiomorphy) or a
new, derived behavior which evolved from
Al
in
both groups (i.e.,
either
a synapomorphy
or a con-
vergence). The morphological
differences between
Tetragnatha and Chrysometa are so great that they
have been placed
in
different subfamilies, so syn-
apomorphy appears
to be ruled
out,
and
the
second
hypothesis thus requires convergence. Nevertheless
it seems more likely than symplesiomorphy when one
examines
the
details of the
spiders'
behavior. This
is because A2 seems to be just a modification of
Al
in
which the last part
of
the behavioral sequence is
omitted, and
it is
"derived from"
Al in
the course
of
the construction of each web as is illustrated in Fig.
9.
A
selective advantage for A2 over Al, which could
explain why the proposed convergence occurred, is
easy
to
imagine since
the
turning back and
forth
which is characteristic
of Al
must be wasteful
of
energy, especially
near the hub where the
spider
can
easily
use
the
closely spaced
radii to move more
di-
rectly from one
attachment to the next. It
is inter-
esting
that
Chrysometa
is considered
by
some
(Levi,
1978a) to be near to the ancestral stock of araneids
on
morphological grounds.
The
exceptional
A2 be-
havior of
spider
#2173
(undescribed genus) (all
other
metines and tetragnathines performed Al) may rep-
resent the culmination
of
this process.
A
modification of
A2
similar
to but not the same
as
A3
is
present
in
some
Micrathena
(Appendix 2).
The non-identity suggests a convergence with
A3
behavior
in
Nephilinae
and thus leaves the deri-
vation of
A3
in
the
latter
group
in doubt.
Again
en-
ergetic efficiency
could
explain
the
convergence.
A4
seems
closest
to Al
since both involve the same
orientation of the
spider's body along
the
radius.
A4,
88). Finally, as the spider began to turn to attach, oIV seized RN+1 close
to the
point
oII
had
been
holding
(100) and maintained this
hold until
the attachment
was made.
Leg iIV: stretched
the
sticky spiral
line
just as
it
was attached
to
RN (0) and
then
helped support the spider
as it moved inward along RN and along
the temporary spiral (20-32); then it began pulling out sticky spiral line (e.g., 52-80),
at
first alternating
with oIV and later with consecutive pulls by itself
until the
last pull
ended in the
stretching
of the line
(100)
as it was attached to
RN+1l

1082
WILLIAM G.
EBERHARD
1
v
~~~~~2
oilli
iIV
inner
01V
Oil
lo
Rn+1
4V
7X9
10
FIG. 4. A3 determination
of sticky
spiral attachment point
behavior, illustrated
from films
of
Nephila
clavipes
taken looking at the
ventral side of
a
mature
female spider
as she moved clockwise near the
bottom
edge of a more or less vertical orb. Conventions are as in Figure 3. Body: faced inward the entire
time,
moving only slightly sideways from one attachment to the next.
Leg
oIV: held RN just outside the attachment
point (1); then it followed
olII
to grip
RN+1,
moving laterally
until the lateral surface
of the tarsus contacted
the radius; it was then extended so that the tarsus slid along the radius until its dorsal surface contacted
the
inner loop
of sticky spiral (10)
(or the outer loop of temporary spiral-apparently no distinction was
made
between the two); it then grabbed the radius near the junction and pulled it toward the spinnerets
(15)
as
the attachment was made just
inside this
point. There was
some variation in the amount
the
tarsus
oIV
actually slid along
RN+1
before contacting a spiral. Leg
olII:
held RN just inside the attachment point
(1),

SPIDER
BEHAVIOR AND
TAXONOMY
1083
As ,'4
B
s
A
l
o
-
---c
-
-
-
FIG. 5. Fl
radius
construction
behavior;
new
lines
are
solid. The
spider
moves to
a frame line
along
a
preexisting
radius,
laying
a
new line behind
it
(a).
This
line is attached to the frame
(b),
and
as
the
spider
moves back toward the hub
it
breaks this
new
line
and rolls
it
up
with
the
pedipalps
(c),
laying
another new line behind
it.
This line is
fastened
at
the
hub
(d),
and
the
spider
usually
proceeds
to
lay
another radius without
making
any
hub
loop
attach-
ments.
which
presumably
involves a
memory
of distances
moved inward and outward
along
radii,
might
seem
to be
a
very
specialized
character,
but
in
fact a sim-
ilar
kinesthetic sense has been
noted
in
two
groups
of
spiders
unrelated
to orb weavers
(Gorner,
1966;
Seyfarth
and
Barth,
1972).
In
addition,
modifica-
tions of
the
experiments
of
Hingston
(1927)
show that
some
orb
weavers which
ordinarily
perform
Al
or
A2
also
appear
to have the
ability
to
sense
and re-
member these distances
(Eberhard,
unpubl.);
and A4
seems to have evolved
convergently
in
the
araneinine
r-4
FIG. 6. F2 radius
construction;
new lines are sol-
id. The
spider interrupts hub loop construction (or
in
other
cases, temporary spiral construction) to
move to the frame along a preexisting
radius,
laying
a new line behind it which it
holds with one leg IV
(a).
This line is
attached to
the
frame
(b),
and then
the spider moves farther along the frame and at-
taches it
again, maintaining
its
hold on the first new
line
with
leg IV (c). Finally
it
returns to the hub,
laying
a second
line behind
it.
As it moves it slides
legs
III
along the first new line, and when they en-
counter
the
junction
between this
line and the hub
or
temporary spiral loop,
the
spider
attaches the
newer radius
to the other and resumes hub construc-
tion
(d).
This
description
is the
same as that of
Hingston (1922) of
N. maculata
behavior in all de-
tails
except
his
claim that when
the first new
line
is
attached to the frame one of the
legs
IV
holds the
radius which the spider walked along from hub to
frame.
then
moved to grab
RN+,
(7) and held
this radius
and pulled it
toward the
spinnerets as the attachment
was
made.
Leg iIV:
stretched the current
segment as it
was attached to
RN (1), then
was inactive
until stretching
the next
current
segment (15, 20)
before its
attachment to
RN+,.
Leg oII:
moved
infrequently and was
apparently used
mainly
for
support
by mature
females.
Immature individuals,
however,
directed
it
retro-
laterally and tapped
toward the inner
loop of sticky
spiral before
most but not all
attachments.
Contact with
the
inner loop was made
several
radii
in
advance of
the one
where the attachment
was about
to be made.
The
leg usually
tapped until it hit the
inner loop;
seemingly in
contrast with leg
oIV, it seemed to
distinguish
sticky
spiral
from
non-sticky lines such
as
radii
and
temporary spiral.
The movements
typical
of
immature
N.
clavipes were also
executed by
mature females
of N.
maculata and Herennia
ornatissima,
and an im-
mature
Nephilengys
malabarensis
(mature
individuals of this last
species were
not observed).

1084
WILLIAM G.
EBERHARD
FIG. 7. F3 radius
construction;
new lines are sol-
id. The spider interrupts hub
loop
(or temporary spi-
ral) construction to move to the frame along a preex-
isting radius, laying a new line behind it (a). It
attaches this line to the frame (b) and then moves
back
to the hub along the new
line,
laying a second
line as it goes
(c).
This line is then attached to the
other radius
bounding
the
sector where the new lines
are
laid,
and
the spider resumes hub loop (or tem-
porary spiral) construction (d). Some
radii
laid this
way were attached to each other part way along their
length (e) by Mecynogea sp. (#1040).
genus Cyrtarachne
which
spins
reduced
orbs. Thus
the relationship of
A4
to
the other character states
is not clear.
B, C, D,
E.
There is no a priori way
to
distinguish
primitive from derived states
in
these characters.
G, H, J.
On
the basis of
simplicity, G1, H2,
and
Jl
might seem primitive with respect to alternative
states.
In
each
case
however these
simpler
states
are
only
omissions of
given behaviors,
and
would
thus
be
easy to
derive from other states.
Thus there
is
no
a
priori certainty
of direction of evolution
in
these
characters.
F. F
1
might seem derived with respect to the other
states since
it
involves the
complex
behavior
of
breaking
and
rolling up
one
line while
laying
another
behind rather than
simply walking
under an
unbro-
ken
line. Essentially
identical behavior
is
known,
however
in
non-orb-weaving species
such as
Pho-
roncidia pukeiwa (=Ulesanis pukeiwa) (Marples,
1955a), P. studo (Eberhard, 1981a), Argyrodes
attenuatus
(Eberhard, 1979), Synotaxus
turbinatus
and
two
Chrysso species (pers. observ.) (all
in the
araneoid
family Theridiidae).
Indeed
it
is not
far
g
~~~~~~-
---;----
----
----
b
C~
FIG. 8. F4 radius
construction;
new lines
are
sol-
id. The
spider interrupts
hub
loop
construction to
move to a frame
line
along
a
preexisting
radius,
lay-
ing
a new line behind it.
This
line
is then
attached
to the frame
(b),
and the
spider
lays
a second
line as
it returns to
the
hub.
This
line
is attached to the
first,
thus
doubling
it,
and the
spider
resumes hub
loop
construction
(from
Eberhard,
1972).
The first
radii
laid
(during
frame thread
construction)
were
exceptional:
they
entailed little or no hub
thread con-
struction,
and
after the
frames
were
complete
or
nearly
so,
the
spider
removed the accumulation of
threads
in
the center of the web
and
proceeded
to
begin
"normal"
radius construction
as illustrated
here.
from
the
behavior used
by
spiders
of
many
families
to ascend their
safety
lines after
dropping
from
some
support
above. Thus
there
is no
clear a
priori
way
to
distinguish
primitive
from derived.
Arguments
supporting
the
probable
derivation of
F3
from
Fl1
are
given
in
the text.
I. It
seems
likely
that
Ii
is
primitive
with
respect
to
12
and 13. This is because attack
wrapping
is a
complex
behavior
generally
associated with web
building,
and
lack
of
this
behavior
is
widespread
and
is
typical
of
groups
which are
undoubtedly
primitive
with
respect
to the
groups
discussed here
in
many
characters
including
web
building
(Robinson,
1975;
also
Eberhard,
1967;
Rovner and
Knost, 1974;
Rob-
inson
and
Lubin,
1979).
Due
to
the demonstrated
advantages
of
attack
wrapping
in
subduing
large,
powerful
prey
(data
summarized
in
Robinson,
1975)
it
is also
unlikely
that once
acquired
it
will be lost.
The
only
possible
exceptions
would
be
in
cases
in

SPIDER
BEHAVIOR
AND TAXONOMY
1085
RN
RN+1
RN
RN+1
FIG. 9. A
diagrammatic
representation
(horizontal
web
seen
from
above)
of the transitional
Al-A2
determination
of
sticky spiral
attachment
point
behavior of
Tetragnatha
sp. (#2043).
Only legs
I
are
shown.
Leg
oI
was extended
along
RN+1
prior
to
grabbing
it
(solid
drawings).
If,
as
in
A,
it
contacted the inner
loop
before
grabbing
the
radius,
the
spider
immediately
swung
to face inward and attached the current
segment
of
sticky spiral
(dotted lines),
performing
an
A2
sequence
of behavior.
If
it
grabbed
the radius before
contacting
the inner
loop (B),
it turned instead
to face out
RN+,
and used
iI
to
locate the inner
loop (dotted
lines
in
B),
performing
an
Al
sequence.
Occasionally
the
spider began
to
swing
its
body
to face outward
before it had finished
extending iI,
and arrested
this
movement when that
leg
contacted
the inner
loop (A2
"tinged"
with
Al).
Thus
A2
appeared
to be
derived from
Al in
that it consisted of a
part
of normal
Al
behavior,
and
vestigial
Al
movements were sometimes
included
in
an
A2
sequence.
Al
was
performed
consistently
near the
edge
of the
web,
and
was
gradually
replaced by
A2
as the
spider
moved inward.
which most
or
all
of
the spider's prey
were relatively
harmless
(e.g., non-stinging
or non-biting)
and/or
either extremely
efficient
or extremely
inefficient
in
escaping
from the web.
A
possible example
of
the
former are
the bolas spiders
and their
kin which
ap-
pear to specialize
on moths (McKeown,
1952; Clyne,
1973; Robinson
and Robinson,
1975; Eberhard,
1977);
none of these
spiders attack
wraps (but
it is not clear
if this represents
a secondary
loss-see
caption to
Fig. 1 of the
text). An example
of the latter
type are
theridiosomatids
which only
occasionally
attack wrap
and which seem to capture
mostly small
nematocer-
ous flies (J.
Coddington,
pers. comm.; pers.
observ.).
It should
be noted that
acceptance of
the ideas that
attack wrapping
in
araneids
is derived and that once
acquired
it will not be
lost except
in the cases just
described
requires that one
consider
the evolutionary
origin of
attack wrapping
in
the
non-orb-weaving
araneoid family Theridiidae to be independent of
that
in
Araneidae (unless, as
noted in
the text, one
accepts the improbable loss of orb webs in theridiids).
This is not unreasonable since theridiid attack wrap-
ping at least often
involves the use
of
a
type
of
silk
(from the aggregate gland) which is never used in
this way by araneids (this gland's product constitutes
the sticky spiral),
and
because prey wrapping
seems
to have evolved
independently
in
a
number of
spi-
ders (see text). It is interesting to note
in
this con-
nection that the different combs on legs IV used in
wrapping
in Theridiidae and
Uloboridae argue for
a
separate origin
of
wrapping
behavior
in
these
fam-
ilies (the combs are on different segments and formed
by modifications
of
different structures-see Opell,
1979; pers. comm.) (see Eberhard, 1979, for evidence
that such combs
do indeed
function
in
wrapping be-
havior).

1086
WILLIAM
G. EBERHARD
c
'i-4
.
=
(1) E E
-5
:;;
0
- =
9
0
0
S.-
;.4
--d
m u
z
(u
-
Cd
u
(U
cd
Cd
W
$..4"
4
cd
>
(U
>
U
'o
(U 'w
0
0
0
'IC
.-
=
--I
::I
cd
c
'n
u u
o ::I
7
o w
(U
(U
-Z
-
W
Cd
Cd
w
+-J m
in.
,
14
d
cn
:
Cd
m
(U
;zq
cn
i--j
w
bo
o
4.5
00
bo
M
-Cs
-z
0
Cd bo
0 CZ
0
(n W
4-4
V
- o g.-
(u
o o
-C$
w
>
0
4--j
U)
Cd
bo
$:
>,
un
Cd
cd
$.4
Cd
ts
7s
-C$
0
bo
4-j
0 4-i 4-i
ce
w
g M
4.5
4-j
+-j j..j Cd
Cd
cd
Cd
0
0
Cd
CZ CZ CZ
CZ
Cd
W
$
w
w
-Cs
bo
9'.
bo
w
bo
$4
0
1.4
(u
-4-J
+-j
(n
>
cn
0
<U
(U
w > E
Z
s. -
>, =
<U
4--j
Cd
u
1:1.
-4
S
-,C
$.4
= E
u
o
cn
:
cn
Cd
00
00
oo
M
CIA
cn
cn t-
00
C', C',
C'S
cd
cri
-C$
u bo
bo
o
cn g
9
CZ
(U ;
5
M -C$
0
.-
E
"..,
0.
u
-C$ ::I
E
(U
0
u
U)
<U
U)
4--4
(u
o
(u
cd
cn
bo
-;::I
cd
(u
crj
>,
0
4-J
0
0
Cd
0
Cd w
tzx
w
>
s.
+-j
0
4-j
+-j
(n
u U w
w
cn
a
cd
0
cd
0
> Cd
9
cd
u
4-4
E u
0
cd
(u
0
-C$
0 =
$.4
r.
+-J
4-j
cd
-j
Cd
t4..,
aj
4-)
Cd
0
0 u g
'r
0
cn u
.
0
0
0
'Ci
E ,
>,
" -C$
bo -c$
-,
bo
.td-
cd
0
g
cd
cd
Cd
M
cd
Cd
x
Cd
Cd
x
s-4
-C$
r.
-Cs
i--j
.1 -
bo
bo
cd
0 0 0
4-j
<U
tn
cn
cn
U
t:
4-j
,M
0
bo
'r.
(n
i..J .-
W
U
(n
tz
Cd
u
cd
g
cd
-,t
Cd
t: ,
cd
>1
cd
g-.
-.4
-.4
0
cd
'r
>
cn
'd
>
Cd
tZ
0
cd
-zi
bo
cd
c
o
bt
0
to
r4
Cd
Cd
bo
9'.
;
bo
oz
Cd
bo
4, u
>
> u
Cd
Cd
bo
W
0
Cd
u Q)
'C$
o
Cd
4
-C$
Cd
Z
cd
g-. g-. (u
::I
$4
Cd
X
M
o
4-j
(u
u o
0
g u
0
cn
C)
u cd
cd
$4
IC$ 0
0
w $4
4-4
i
bo Cd
9
Cd

SPIDER
BEHAVIOR AND
TAXONOMY
1087
C9
C-CI)
CC4
_-
C"9
N
C
N
N
CN
N
C Q _ __ _ ___ _ ________ _~~~~~~~~~CI
-
00 00
00
00
0
0~~~~~~~~
cn
~ ~ ~ ~ ~ ~ ~ ~ ~~~~0
_0 __
_ _.
_ _
_
_ _
_
_ _ _
_ _
_
<^
Z 0
_ __
__
_
__
_
_
__
___
__
__
t
.E N~~~~~~~~~~~~~

1088
WILLIAM
G.
EBERHARD
U-)
10
10
C',
C',
en
en
i-
-)
Cj)
Cen
C-
-ai
00_
00000
_
_
_
-~C-c
_4
_ _
00
S Q
0)
m
_
_ _
_
_
_ _
_
_
_
_
_
c9
c9
_
_
cL
C;~~~~~~~~~
t-t
X c c O O e e t e _ = c e cro X
O~~~~~~~~~ajce
~0) C 0~-0~ o
0)0) Ch 0h I 1 c9 c9 Ch ~ Ch Ch ?O > C.
c
z

SPIDER
BEHAVIOR
AND
TAXONOMY
1089
In
tO tO
C
r-,
-r,
r-
--
in
\C
r- ON (N
06
,O
c
m ~ ~ ~ ~
\
n
\C
O' C ON
0
N cni
C
0
c9
C9l
\C
_- I'_
r-O,l-0
00
00 00 00
C.9
,,I-c9c9
c9
c
c9
in
-c9
r9
in
t9
c c9
-
',I
in 00
0909c9
X
Q
o ~ ~ ~~t-
tt
t9
o
.0
0
C
'00
0
oo
s
t:t
t:t
( t- t
't-
4z
(U
t:t 0 DeO
C~~~~~~~~~~~~~~~~~~~~)
~ ~ ~ ~ ~ ~ ~ ~ '
X
_ c _
e c O
_ c O
% N e
m c %
e _ N
e N c
~~~~~~~~~~~~~~~~t:Ot
C
%
0~~~~~~~~~
?~ ~~
a
C/)
Y
00X
t:t
C~
~~~s
S
-~~~tt
's;
=~~~~~~~
ol)
~ UC~
00
C
.
V
C'S

1090
WILLIAM
G.
EBERHARD
(
eeN (N (N (N (N (N
c-
c-
c-
N
CN
C"
C"
C"
C"
-4
C"
eN
C'
c
C" C"
Cl
C)
C"l
C"
C"
C"l
CN CN
CN
CN
cl
r
l
C)
N
CN CN
C
N
C)
"
"
C"l C"l
C"
CN
CN
CN Cl
0~~~~~~~~~~~~~
.on
0
N
(zN(N (N(N(NC N
(N(N(csN
(9N(N
(N (N ~N
(N
(N
00
-
~ ~ ~ ~ 00(N C~~~~~t'.r-00
in
i
' '
-0
0
,-
,- o
0
i
0N
00
_l
U
00
CZ
-0
w
C
>
N~~~~~~~~~~~~~~~(
u
=
~~~~~c
v
~
~~~~~~~~~~~~~~~~~
C
oR
E~~~~~~~~~~~~~~~~~~~~~~~E
,~~~~~c
Q
1>,
2
r aS o

SPIDER
BEHAVIOR AND TAXONOMY 1091
C"
(
eeN (N (N
(N
(N (N (N
cl-
cl-
cl-
c
g" g"
C"
g"
g
Q
C"l
g" g"
C" C)l C)l (N gN(
N(
0~~~~~~~~~~~~~0
(
m
~~~~~N (N (N (N(N N(N(N(N(N(N N
CN (N
az
~ ~ ~ ~ ~ ~ ~ ~ ~~~r
\C -.-I in
0
0
N(NN
(N (N (NN
(N(NN
C(N(N
(N
~tt
(N
00~~~~~~o
t~~~~~~
t~-CC W
D OOi ;O00 tk
00~~~
Z
~ ~ 0 N-
N
)
<N-
\
U)
00
t
U)
eX
C ~~~~~~~C
(u
( L
;
=N
SX
O
-
-Z!
0
U~~~~~~~~(

1092
WILLIAM
G.
EBERHARD
C"
C"
C"
cn
n cn
n
cn
c-
C"
(N (N
n cn
1- 1- --_-
v
~ ~ ~ ~ C l
CZ
Ca Ca Ca
Ca CZ
CZ
C
0n0
t ~t
0
~ " o
a-
_ln
00
C' -4
00
in
r-
-4r
4-
0~~~~~~~~~~~~0
*>~~~~~~~~~~~~~0
0
-6
d
m
0
Cl)
0 ~~~~~~~~~~~~~Cd
l
C
_Id t:t em NNNP
X .s O c ? .M 0
t~~~~~
=~
H i j 9 W - w i 2 X ' E E . t
. cX-Z
EH
?
>

SPIDER
BEHAVIOR AND TAXONOMY
1093
=4
g
4)
44, cd
'o
>
C's
4.)
>
>
C,3
C's
a) , C's
C's
bi)
r-
Z
4) biD
Cd
0
-a
=-
-Zj
biD
r.
C's
C's
0
0
ti
u
C13
6
C13
NO
Z
0
0
0
E.
0
"o
0
C13
Z
'A
CU
r.
0
>
0
>
C13
C's
0 u
a7l
r.
a
v
0
-
C
4.)
4.)
0
pq
1-4
cl-I
iz
ln
=
0
4.)
>,
bl)
C's
0
0
-1
C13,
X4
4.)
C's
u
u
M
(D -t
.-
-
:
4.) "
r.
C's
0
r.
CN
C's -.C
o
06
m
Z 0
C13
o o
cd
C's
>
C's
-
=
I..
r.
r.
z
>
0
Z
Z
C's
o
0
Z
a
>
CIS
0
Cd
0
biD
z
-o
q,-
0
0
0
bi)
bO
C's
r.
0
o
-d
r.
C's
co
C's
0
Z
CN
-bl)
0
0
>
0
U
-
7--
C,3
0
C'S
Z
-I..
1
-m
:3o
Z
In
'o-
C13
0
0
Z
-.4
m
0
'3
4.)
U
> > >
U)
-
4
Z3
U!
0
In
4) -
= 'n
iz
0
I-Cd
r
7
:6
4)
-
_
:
'(5
0
o
0
cd
C13
C's
bt
C's r-
o
U 4.)
0
a.
o
>,
-
;..
=
4
cn
r. Cd
Z
0
0
U
0
Z:,
'C.-
cd
0
".
a
In
Cd
r.
r-
-Zx
-
In
bO
Cd
Wu
u-m
cl
m
Z
Z
w
CN
r
Wc
p
0
Cd
v
10'
M -
z
=
-
r.
In
o
C)
o
19
-
.2
E
r,
P4
0
0
P4
a
r
Cd
0
C,3
0
Cd
cd
4.)
b
0
4.)
U - 4.)
C,3
-i5
.-
U
,
C's
M
'5
: 'o
=
4.)
:6
-
C,3
r
im.
C)
0
C,3
U)
E
0 r
M
r.
g
r
4.)
-
0
K
C'3
c
-0
0 C%
E;a
:
-a
.
0
7
0
M.
0
In
0
C)
cd
bo
Cd
0
Cd
cd
CIS
E
'o
C's
'o
A:
Cd
4.) 0
M 0
cd
C,3
4.)
M 0
r
>
0
I..
>
bO
4.)
r.
.0
0
C13
r.
r.
z
-,;5
U
.-.z
:
0
c)
=
0
r.
.0
C)
bO
0
im.
tn
E
c)
v
z
0
>
C13
=s
C,3
U
0
O 0
r.
c
C13
0
0 0
M W
w
C-1 3
C,3
r-1. C,3
14 0
CZ
0
M
biD
>
bl)
W
0
>
o
4)
-
>,
r.
W)
0
0
>
0
0
'n
4.)
-
.-
4.)
zls
r. r-
C,
>
o
C13
>
0
0
>
M
0
v 0 v
-
0
0
1.
n
'o
0)
C13
r-
>
C)
CI.
o
Cd
0
O
0 0-
0
cd
a :3
r-
C13
M
04
n
$M.
v
4.)
.
-
(:, :.-Z
0.-
0 -
r
>
0
4.)
.0 >,
a
C'S
bO
,
n
-
'o
Z7
C13
0
-w
r
0
Z
c)
-.4
0
>,,=
U
7
C,3
>,
aq-,
C,3
c)
o o
C,3
'n
7
-
.Z
: -
a
0
Cd
0
zu
v
w
a
>,
=
,,
-0
r.
't
cd
0
o a IL)
4
b,
Z3
0 -
w Z 'n
w
=
0
Cd
o
0 ,
.
-
$M.
'S
0
0
'0'
-
'n
"
cd
>
0
'o
r.
bj)'-
Cl 0
'n
U
cd
0 CIS
'o
>
C13
;>
a
a
_A
C's
$M.
14
0 o o o
o" o
C13
v
C)
r.
U) a P4
-
-
-
=
,
r.
$..
-
C13
o
En
>,
4)
r
M 0
bO
Cd
r.
',;
4.).-
r
b
0
O
O'
M
7
>
bll
4.)
=s
r r
0
bO
r
r
ql)
M
0 0
0
c,3
o
C'3
bO a
Z
C13
C13 o C13
r,
C, 34i l) r,
C)
-0
bb,,
(:)
-
4
-
.- C-
:
-
-
Z
.-
U
0
4j
4.)
T.
U
C,3
0
cd
:Y-
r
:3
:3,0
biD
4.)
0
v
U
v
v
0
I...
5.
(::)
4.)
o
C,3
0
To
2
P.
0
ar
r:
=
-1
-
0

1094 WILLIAM G. EBERHARD
APPENDIX
3.
Attack behavior of at least 81 species in at least 38 genera.
Observations with
no source indicated are
original,
and numbers refer to
descriptions
in
Appendix
1.
It
should be
kept
in
mind
that
"4"
is not a
separate category,
but rather
indicates ignorance
of
whether
the
character state is
2
or 3.
"( )"
indicates that less than 6 attacks were
observed, and applies only to original
observations.
Spider
Attack
behavior Source
Araneidae
Tetragnathinae
Dolichognatha spp. #533 2
#0-2
2
Metinae
Leucauge sp. near venusta 2
L. spp. #527
2
#526
2
#0-21-5
2
Chrysometa spp. #532 2
#0-6
(2)
Nephilinae
Nephila clavipes
1
Robinson,
1975
N.
maculata
1
Robinson,
1975
N.
constricta
1
Robinson,
1975
N. turneri
1
Robinson,
1975
Nephilengys
cruentata
1
Robinson,
1975
Herennia ornatissima
1
Robinson, 1975
Araneinae
Argiopeae
Argiope argentata 3
Robinson and Olazarri, 1971;
pers.
observ.
A. savignyi 4
Robinson, 1975
A
florida
4
Robinson,
1975
A. aurantia 3
Robinson, 1975; pers. observ.
A. aemula 4
Robinson,
1975
A. picta 4
Robinson,
1975
A. ocyaloides
3
Robinson and Lubin, 1979a
A. sp. 4
Robinson, 1975
Cycloseae
Cyclosa caroli
3
C.
conica
3
Marples
and
Marples,
1937
C.
triquetra
3
C.
furcata or bifurcata
3
Salassina
sp. #2226
3
Mangoreae
Mangora melanocephala
3
M.
sp. #1569,
1641 3
Acacesia hemata
3
Cyrtophora cylindroides
4
Lubin,
1973
C.
monulfi 4
Lubin,
1973
Eustalafuscovittata
3
Spilasma artifer
3
Araneae
Araneus diadematus
4
Peters,
1931
A.
marmoreus
4
Robinson,
1975
A.
rufipalpis
4
Robinson,
1975
Alpaida leucogramma
3
Eriophorafuliginea
3
Robinson
et
al.,
1974
E.
nephiloides
4
Robinson,
1975
E.
edax
3
Wagneriana sp.
#574
(2)

SPIDER
BEHAVIOR
AND TAXONOMY
1095
APPENDIX 3. Continued.
Spider
Attack
behavior Source
Metazygia gregalis
3
M. sp. #0-21-2 3
Metepeira labyrinthica
3
M. sp. or spp. #254 (2)
#1313
3
Wixia
ectypa
2a
Stowe,
1978
Arachnureae
Arachnura melanura 3 Robinson and Lubin, 1979a
Hypognatheae
Hypophthalma sp.
3
Gasteracantheae
Gasteracantha
cancriformis
lb
Muma, 1971; pers.
observ.
Micratheneae
Micrathena clypeata
1
Robinson,
1975
M. schreibersi
1
Robinson,
1975
M. sexspinosa
1
Robinson,
1975
Mastophoreae
Mastophora dizzydeani
1
Dichrostichus
magnificus
1
Longman,
1922
Cyrtarachneae
Poecilopachys australasia
1
Clyne,
1973
Pasilobus sp.
1
Robinson and Robinson,
1975
Celaenieae
Celaenia excavata
1
McKeown, 1952
Taczanowskia sp. (1) Eberhard, 1981b
Theridiosomatidae
Olgulnius spp. #1292 (1)
#EG2-22III76
(2)
Epeirotypus spp. #1093 (1)
#1054
(1)
#1603 (1)
#802 (1)
Uloboridae
Uloborus walckenaerius
2
Marples,
1962
U. congregabilis
2
Marples,
1962
U. diversus
2
Eberhard,
1967
U.
trilineatus
2
Philoponella semiplumosa
2
P. republicana (2)
P.
tingena (2)
P.
vittata
(2)
P.
oweni
2
P. arizonica
2
P. para (2)
P. sp. nearfasciata #Moz-14
2
Zosis geniculatus
2
Marples, 1962, pers. observ.
Hyptiotes paradoxus
2
Marples,
1962
Miagrammopes simus
2
Lubin et al.,
1978
M.
intempus
2
Lubin et
al.,
1978
M. sp. near unipus (2)
Lubin
et al.,
1978
a
Prey
is
wrapped
on branch
rather
than
in
web, spider's
web
is so
unusual that
comparisons
with
orb weavers are
probably
not
justified.
b
Robinson (1975) attributed
attack
wrapping
to
this
species
Muma's
account,
on
which
Robinson
based
his
decision,
is
unclear
and
is
open
to
other
interpretations (Muma, 1971) My
own
observations
(20
attacks
on a
variety
of
potentially dangerous [wasps,
bees]
and
innocuous
prey
suggest
that this
species
does
not
attack wrap.
Robinson later
(Robinson and Lubin, 1979a)
included
Gasteracantha
in
a
list
of
genera lacking
attack
wrapping.
